Avicenna Journal of Clinical Microbiology and Infection. 10(4):166-172.
doi: 10.34172/ajcmi.3505
Scoping Review
Carbapenem Resistance among HIV-Infected Patients: A Scoping Review
Kylie Divashnee Konar 1  , Rushern Ruvashin Chetty 2, *
, Rushern Ruvashin Chetty 2, *  , Selina Konar 3
, Selina Konar 3  , Somasundram Pillay 4
, Somasundram Pillay 4 
Author information:
1Department of Internal Medicine, Frere Hospital, East London, South Africa
2Medical Doctor in Private Practice
3Department of Microbiology, Inkosi Albert Luthuli Hospital, Durban, South Africa
4Department of Internal Medicine, King Edward Hospital, Durban AND Department of Internal Medicine, University of Kwa-Zulu Natal, Durban, South Africa
Abstract
Background: Antibiotic resistance is an escalating global health concern, with carbapenems, potent last-line antibiotics, facing increasing resistance and potentially dire consequences. This scoping review sought to consolidate data on carbapenem resistance in human immunodeficiency virus (HIV)-infected patient cohorts as the intricate relationship between HIV and antibiotic resistance remains inadequately understood.
Methods: We employed a scoping review methodology and conducted a comprehensive search across Google Scholar, Scopus, Cochrane, and PubMed, utilizing specific search terms related to carbapenem resistance and HIV. We extracted and analyzed data, encompassing study design, geographic location, number of HIV-infected participants, CD4 cell counts, specimen types, cultured organisms, carbapenem susceptibility, and comparisons between HIV-infected and uninfected cohorts.
Results: This review encompassed 15 studies, involving 2365 HIV-infected participants, primarily employing cross-sectional designs, with nine studies conducted in African countries. The most frequently analyzed specimens included urine, stool, and sputum, with Escherichia coli emerging as the most frequently cultured organism. Commonly used carbapenem drugs included imipenem, meropenem, and ertapenem, with varying susceptibility patterns. Imipenem and meropenem exhibited sensitivities exceeding 80%, except for one study with Pseudomonas aeruginosa, which demonstrated 73% sensitivity. Ertapenem displayed fluctuating sensitivities ranging from 58% to 100% for different bacterial organisms. Only one study reported the colonization of carbapenem-resistant Enterobacterales (CRE) in HIV-infected patients, with HIV status not significantly influencing CRE carriage. When comparing HIV-infected and uninfected cohorts, four studies found no substantial impact of HIV status on carbapenem resistance.
Conclusion: In the context of the HIV burden and opportunistic infections, carbapenem resistance demonstrated relatively consistent patterns across most studies comparing HIV-infected and uninfected cohorts. However, the presence of CRE among HIV-infected individuals raises concerns regarding nosocomial infections. The limited reporting of CD4 counts in the included studies necessitates further exploration of potential associations with immune status. E. coli, frequently cultured in these studies, exhibited varying resistance patterns, and the impact of HIV on these patterns remains uncertain. Carbapenem susceptibility displayed variability among different organisms, underscoring the nuanced nature of resistance. As such, this scoping review serves as a foundation for comprehending carbapenem resistance in HIV-infected populations but underscores the necessity for more comprehensive research in this field.
Keywords: Carbapenem, HIV, Resistance, Scoping review
Copyright and License Information
© 2023 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Please cite this article as follows: Konar KD, Chetty RR, Konar S, Pillay S. Carbapenem resistance among HIV-infected patients: a scoping review. Avicenna J Clin Microbiol Infect. 2023; 10(4):166-172. doi:10.34172/ajcmi.3505
Introduction
Antibiotics, which play a pivotal role in the management of bacterial infections, are susceptible to resistance when bacteria develop altered responses to these therapeutic agents (1). Notably, Shallcross et al reported that approximately 30.1% of patients receive antibiotic prescriptions annually, with primary care accounting for nearly 50% of antibiotic prescriptions, targeting a population of less than 10% of patients (2). The consequences of antibiotic overuse encompass an increased risk of adverse effects, higher rates of healthcare utilization, and augmented medication consumption for self-limiting conditions (3). The most recent global statistics indicate that antimicrobial resistance (AMR) leads to over 700 000 annual deaths (4).
Various categories of antibiotics are available, and in the face of mounting concerns regarding AMR, the World Health Organization (WHO) has identified a group of pathogens collectively known as ESKAPE pathogens. These pathogens include Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species. These microorganisms pose significant threats to human health, with many exhibiting multidrug resistance, thereby creating substantial challenges in clinical practice (5). Remarkably, the effectiveness of numerous antibiotics has diminished over the decades since their initial introduction, and resistance is now becoming pervasive across virtually all classes of antibiotics (6).
Carbapenems, possessing distinctive pharmacological characteristics, are frequently prescribed for the treatment of complex bacterial infections (7). They are widely regarded as the most potent antimicrobial agents in combating the most stubborn bacterial strains (8), often recognized as the appellations of “last-line agents” or “antibiotics of last resort” (7). Consequently, any decline in their efficacy represents a serious threat to human health. Rima et al have documented a significant increase in the prevalence of carbapenem resistance among Enterobacterales, rising from 1.4% to 3.3% between 2015 and 2019. A similar trend was observed in Pseudomonas species, where resistance increased from 8.1% to 27.3% during the same period (9). A study conducted in 2022 examining carbapenem resistance in the Netherlands revealed that while resistance in Escherichia coli and K. pneumoniae remained relatively low, the incidence of carbapenemase-producing Enterobacterales surged between 2017 and 2019 (10).
Human immunodeficiency virus (HIV) is a global viral infection, affecting approximately 14% of the population in South Africa (11). HIV substantially increases susceptibility to opportunistic infections, with bacterial infections being the most prevalent among them (12). As a result, individuals living with HIV often need an increased use of antibiotics, potentially raising the risk of antibiotic resistance emergence. Of particular concern is the possibility of resistant strains spreading to individuals who do not use antibiotics (13).
Objectives
The primary objective of this scoping review was to compile and synthesize data pertinent to carbapenem resistance from articles that specifically focused on cohorts of individuals infected with HIV.
Methods
Scoping reviews are utilized to assist authors with evidence-based synthesis of resources to help plan and conduct their research (14). Westphaln et al describe five steps that can assist with such a review: steps one to three involve identifying the research question and identifying the relevant literature with the selection of appropriate studies (15), and steps four and five are to extract and chart the date and summarize and report the results (15). The authors utilized this structure when preparing this review.
The methodology for determining resistance was based on the criteria used by individual articles. In this scoping review, we analyzed the results of previous articles without questioning their methodology as each utilized article has previously been peer-reviewed and published.
Search Terms and Data Sources
The search engines utilized for this scoping review were Google Scholar, Scopus, Cochrane, and PubMed, and the following search terms were utilized: “Carbapenem Resistance” OR “Imipenem” OR “Meropenem” OR “Doripenem” OR “Ertapenem” and “HIV” or “AIDS”. The full form of “human immunodeficiency syndrome” or “acquired immunodeficiency syndrome” was not looked at separately since articles that mentioned these terms had the relevant acronyms which covered their searches, namely, “HIV” or “AIDS”. Searches were reviewed, and relevant articles had their “abstract” reviewed. If an article met our inclusion criteria, it was kept aside for full review for this study. A brief ‘Google Search’ was also conducted by the name of each carbapenem to find additional articles relevant to this study.
Any original research article that mentioned an association between carbapenem resistance in a cohort of HIV-infected adult patients was included in this scoping review. Moreover, no restriction on the year of publication or country of publication was placed in our results.
The articles excluded from this scoping review included non-English language articles, case reports, case series, studies focussing on cohorts of tuberculosis, pregnancy, pediatric patients, or literature reviews.
Data Synthesis
On 22 September 2023, a total of 219 articles were identified using the above search terms on the medical search engines (Google Scholar = 55, PubMed = 151, Cochrane = 3, and Scopus = 10).
We reviewed articles by ‘Title’ to see if they are relevant to this study. If it was suitable for the review, then the full ‘Abstract’ was reviewed to see if the article was pertinent. Afterward, the full article was read to assess whether it met our inclusion criteria. If it met the criteria, it was saved for full analysis in our results. A summarized approach to the article selection is found below (Figure 1).
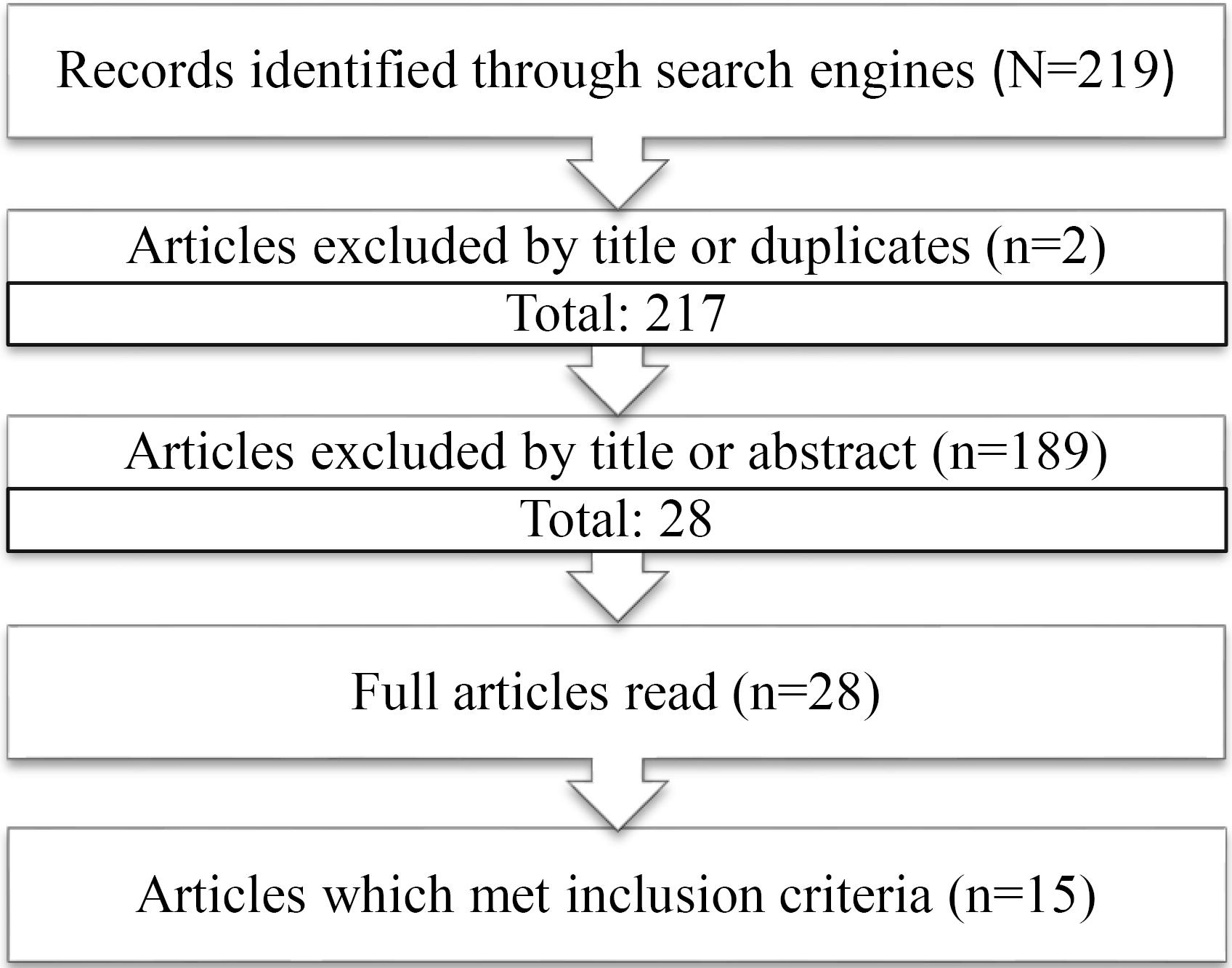
Figure 1.
A Flow Diagram of the Final Selection of Articles
.
A Flow Diagram of the Final Selection of Articles
A total of 15 articles that met our inclusion criteria were selected and summarized in Table 1.
Table 1.
Articles Included in Scoping Review
|
Study Year
|
Author
|
Type of Study
|
Country
|
No. of HIV-infected (%)
|
CD4 (cells/mm3)
|
Specimen Site
|
Hospitalized Patients (%)
|
Most Common Organism Isolated in HIV Patients
|
Associations Present in the Study
|
| 2023 |
Omulo et al (16) |
Cross-sectional |
Kenya |
122 (14.8%) |
- |
Stool |
100% |
CRE colonization |
HIV status was not associated with CRE carriage. |
| 2023 |
Singh et al (17) |
Retrospective observational descriptive study |
South Africa |
64 (19.5%) |
586 |
Bile |
100% |
K. pneumonia (22.2%), Enterococcus
species(22.2%) |
Overall, carbapenem-based therapy, namely, imipenem and meropenem, demonstrated the lowest resistance levels of 6.7% and 2.7%, respectively. No significant differences were noted between HIV-infected vs uninfected cohorts with carbapenem use. |
| 2022 |
Lowe et al (18) |
Cross-sectional |
South Africa |
185 (31%) |
- |
Blood culture |
100% |
CRE K. pneumonia (79.8%) |
HIV status did not significantly affect hospital mortality in patients with CRE bacteremia. |
| 2021 |
Abongomera et al (19) |
Cross-sectional |
Uganda |
200 |
- |
Urine |
0% |
E. coli (72%) |
No resistance to imipenem was observed. |
| 2021 |
Rameshkumar et al (20) |
Cross-sectional |
India |
173 |
|
Urine |
Not mentioned |
E. coli (47.4%) |
43% of Gram-negative bacteria were resistant to carbapenem (imipenem) antibiotics. HIV-infected patients had a multidrug-resistant profile and showed a higher level of resistance to the 3rd generation cephalosporin antibiotics than to carbapenem and cephamycin antibiotics. |
| 2020 |
Tessema et al (21) |
Cross-sectional |
Ethiopia |
224 |
- |
Urine |
100% |
E. coli (69.6%) |
87.5% were susceptible to meropenem. |
| 2019 |
Ngalani et al (22) |
Cross-sectional study |
Cameroon |
100 |
459 |
Stool culture |
100% |
K. pneumoniae
|
All isolates were sensitive to imipenem in both HIV-infected and non-infected patients. |
| 2018 |
Swathirajan et al (23) |
Retrospective study |
India |
260 |
- |
57.3% sputum |
63.5% |
P. aeruginosa
|
Carbapenem resistance among P. aeruginosa isolates had 15.0% (39/260) imipenem-resistant strains and 16.9% (44/260) meropenem-resistant. |
| 2018 |
Yang et al (24) |
Case-control study |
China |
56 |
19 |
Respiratory (89.3%) |
Not mentioned |
A. baumannii
|
Extensively drug resistance and pan-drug resistance A. baumannii were isolated in 16 and 17 patients, respectively, implying that at least 58.9% of AB strains are resistant to carbapenems. This favors the coverage of carbapenem-resistant strains in empirical treatment for HIV patients at risk of A. Baumannii infection. |
| 2017 |
Reinheimer et al (25) |
Retrospective |
Germany |
109 males |
|
Rectal swab |
100% |
E. coli
|
No resistance to carbapenems was detected in any of the isolates from HIV-infected or uninfected men. |
| 2016 |
Chiou et al (26) |
Case-control |
Taiwan |
54 |
348-511 |
Stool |
Not mentioned |
S. Sonnei
|
All samples were sensitive to ertapenem and imipenem. |
| 2014 |
Luyt et al (27) |
Prospective observational |
France |
2 |
- |
Sputum |
100% |
P. aeruginosa
|
One patient was sensitive to carbapenems, while one patient had intermediate susceptibility/resistance when immunosuppression was secondary to HIV. |
| 2012 |
Iweriebor et al (28) |
Cross-sectional |
South Africa |
195 |
|
Urine |
Not mentioned |
Enterobacter species |
The majority of the isolates were susceptible to imipenem. |
| 2011 |
Samie et al (29) |
Cross-sectional |
South Africa |
140 |
|
Mostly stool (42%) |
Not mentioned |
S. aureus
|
Most isolates were susceptible to meropenem (81.2%) |
| 2005 |
Srifuengfung et al (30) |
Cross-sectional |
Thailand |
481 |
|
Sputum |
Not mentioned |
P. aeruginosa (32.97%) |
P. aeruginosa was 73% sensitive to both imipenem and meropenem.
However, only 11% and 5% of methicillin-resistant S. aureus isolates were sensitive toimipenem and meropenem, respectively. |
Note. HIV: Human immunodeficiency virus; CD4: Cluster of Differentiation 4; CRE: Carbapenem-resistant Enterobacterales; K. pneumonia: Klebsiella pneumonia; E. coli: Escherichia coli; P. aeruginosa: Pseudomonas aeruginosa; A. baumannii: Acinetobacter baumannii; S. Sonnei: Shigella Sonnei; S. aureus: Staphylococcus aureus.
Results
Demographics of Different Studies
In this comprehensive review, a total of 15 studies involving 2,365 individuals living with HIV were subjected to rigorous analysis. The majority of these investigations adopted a cross-sectional research design, and notably, nine out of the 15 studies were conducted within the African continent (Table 1).
Specimen Type and Organism Culture
Among the diverse range of specimens scrutinized in the included studies, urine, stool, and sputum were most frequently analyzed, each accounting for four instances. It is noteworthy that E. coli emerged as the predominant cultured organism, featuring prominently in four out of the 15 studies (Table 1).
Carbapenem-resistant Enterobacterales Colonization and Human Immunodeficiency Virus
Within the scope of our review, a solitary study conducted by Omulo et al (16) offered insights into CRE colonization among individuals living with HIV. Notably, this investigation revealed that the HIV status of participants does not exert a discernible impact on the carriage of CRE (Table 1).
Carbapenem Resistance in Human Immunodeficiency Virus
The carbapenem drugs (i.e., imipenem, meropenem, and ertapenem) were commonly employed in the studies conducted among individuals infected with HIV. However, it is important to note that all studies included in our review reported varying levels of susceptibility to these carbapenem agents. Notably, the study by Srifuengfung et al (30) documented the highest resistance rates, with only 11% and 5% of methicillin-resistant S. aureus isolate being sensitive to imipenem and meropenem, respectively (Table 1).
Furthermore, a subset of four studies within our review undertook comparisons between cohorts of HIV-infected and HIV-uninfected patients. Intriguingly, these investigations collectively found that HIV status does not exert a statistically significant influence on carbapenem resistance, as elucidated by Singh et al (17), Lowe et al (18), Ngalani et al (22), and Reinheimer et al (25) (Table 2).
Table 2.
Summary of Antibiotic Sensitivities Described in Studies
|
Study
|
Organism
|
Carbapenem (%)
|
Imipenem (%)
|
Meropenem (%)
|
Ertapenem (%)
|
Association between HIV-infected vs. Uninfected Patients
|
| Omulo et al (16) |
CRE |
|
|
|
|
HIV Status was not associated with CRE carriage. |
| Singh et al (17) |
KP, ENTC, ENT, ESC, CITRO, SA, ESSA, and others |
|
90.9 |
100 |
|
No difference between cohorts |
| Lowe et al (18) |
KP, EC, SM, EC, other |
|
|
|
|
No difference in mortality |
| Abongomera et al (19) |
EC, KP, MM, other |
|
100
All except MM |
|
|
|
| Rajeshkumar et al (20) |
EC, KP, KO, PV, PM, PA |
57 |
|
|
|
|
| Tessema et al (21) |
EC, KP, EA, PA, SA |
|
|
100 EA, KP, PA.
87.5 EC |
|
|
| Ngalani et al (22) |
EA, KP, EC, Shigella species, Salmonella species |
|
100
All organisms |
|
|
No difference between cohorts |
| Swathirajan et al (23) |
PA |
|
85 |
83.1 |
|
|
| Yang et al (24) |
AB |
41.1 |
|
|
|
|
| Reinheimer et al (25) |
EC, KP, PA, AB |
100
All organisms |
|
|
|
No difference between cohorts |
| Chiou et al (26) |
S. sonnei
|
|
100 |
|
100 |
|
| Luyt et al (27) |
PA |
50 |
|
|
|
|
| Iweriebor et al (28) |
CITRO |
|
100 |
|
78 |
|
| Klebsiella species |
|
100 |
|
58 |
|
| ENT |
|
85 |
|
54 |
|
| EC |
|
80 |
|
77 |
|
| Samie et al (29) |
SA |
|
|
81.2 |
|
|
| Srifuengfung et al (30) |
PA |
|
73 |
73 |
|
|
| SA |
|
|
|
|
|
| MSSA |
|
100 |
100 |
|
|
| MRSA |
|
11 |
5 |
|
|
| KP |
|
100 |
99 |
|
|
| HI |
|
9 |
99 |
|
|
| AB |
|
93 |
90 |
|
|
Note. CRE: Carbapenem-resistant Enterobacterales; AB: Acinetobacter baumannii; CITRO: Citrobacter species; EA: Enterobacter aerogenes; EC: Enterobacter cloacae; ENT: Enterobacter species; ENTC: Enterococcus species; ESSA: Enterobacter species Streptococcus anginosus; ESC: Escherichia coli; HI: Haemophilus influenza; KO: Klebsiella oxytoca; KP: Klebsiella pneumonia; MRSA: Methicillin-resistant Staphylococcus aureus; MSSA: Methicillin-sensitive Staphylococcus aureus; MM: Morganella morganii; PA: Pseudomonas aeruginosa;PM: Proteus mirabilis; PV: Pemphigus vulgaris; SM: Serratia marcescens; SA: Staphylococcus aureus; S. sonnei: Shigella sonnei.
Type of Carbapenem Used and Resistance in Human Immunodeficiency Virus
Imipenem and meropenem demonstrated robust sensitivity rates, exceeding 80% in the majority of studies, with the exception being P. aeruginosa in the study conducted by Srifuengfung et al (30). In this specific study, both imipenem and meropenem exhibited a sensitivity rate of 73% for P. aeruginosa isolates.
In the case of ertapenem, the studies included in the review portrayed a wide spectrum of sensitivities, ranging from 58% to 100% for various bacterial organisms. This variability underscores the nuanced susceptibility patterns observed with ertapenem across different contexts and pathogens (Table 2).
Cluster of Differentiation 4 and Immunosuppression
Among the studies included in the review, four studies provided commentary on a cluster of differentiation 4 (CD4) levels. Three out of these four studies reported CD4 counts exceeding 100 cells/mm3 (Table 1).
Hospitalized Patients
Forty-seven percent (47%) of the studies were conducted among hospitalized patients, while one study (7%) was exclusively conducted in outpatients. Six studies (40%) did not mention whether the cohort of patients were admitted or not, while one study had both inpatients and outpatients.
Discussion
A comprehensive evaluation of carbapenem drug susceptibility, based on a compilation of different studies, paints an encouraging overall picture. The majority of these studies were carried out in African countries, with a noteworthy concentration in South Africa. This concentration can be attributed to South Africa’s position as the epicenter of the HIV pandemic, characterized by the highest prevalence of HIV infection (31), as well as its relatively superior healthcare resources compared to other African nations (32).
In light of the substantial burden of HIV and the associated prevalence of opportunistic infections, resistance patterns of carbapenems exhibited remarkable consistency across most studies that compared patient cohorts, differentiating between those infected with HIV and those who were not. This uniformity in resistance patterns holds significant implications, particularly considering that pathogens can traverse various transmission routes, and HIV-uninfected patients may be susceptible to acquiring aerosolized pathogens from diverse sources, including HIV-infected individuals (33). Notably, a study by Omulo et al reported the presence of CRE among HIV-infected patients, raising concerns about the heightened risk of subsequent infections during their hospital stays (34). This is exacerbated by the limited availability of antibiotics for treating such infections, underscoring the gravity of CRE acquisition (35).
Many of the studies included in this review did not provide information regarding the CD4 counts of HIV-infected patients. It is worth noting that lower CD4 counts have been linked to increased rates of virological failure and drug resistance in antiretroviral therapy (36). Additionally, a study by Liu et al emphasized the connection between lower CD4 counts and elevated resistance to HIV drugs (37). The absence of this specific data point in our review may be attributed to the limited number of articles that reported CD4 values.
Escherichia coli was the most frequently cultured organism in numerous studies. Although E. coli typically exhibits susceptibility to a wide range of antimicrobial agents, it has a notable tendency to develop resistance over time (38). A study conducted in 2019 on E. coli’s resistance to carbapenems revealed that approximately 30% of isolates display resistance to at least one of the three tested carbapenem antibiotics, namely, meropenem, imipenem, or ertapenem (39). Within the studies covered by this scoping review, the impact of HIV on these resistance patterns was not consistently elucidated. The current review identified imipenem, meropenem, and ertapenem as commonly used carbapenems, each exhibiting varying resistance patterns. In most cases, imipenem and doripenem showed effectiveness against Gram-positive organisms, while meropenem, ertapenem, and doripenem were effective against Gram-negative organisms (7).
A. baumannii was also noted for its significant resistance to carbapenems (24). Carbapenem-resistant A. baumannii (CRAB) has gained global notoriety as a significant nosocomial pathogen (40). The limited range of antibiotics that are effective against CRAB underscores the pressing concern of limited therapeutic options when dealing with emergent-resistant organisms, with colistin and tigecycline offering uncertain efficacy (40). This predicament has concerning implications for patients at large, given the limited choices available for managing such challenging infections
In sum, this extensive scoping review has yielded valuable insights into the scenario of carbapenem resistance among individuals infected with HIV. Carbapenems, acknowledged for their potency as antimicrobial agents, serve as pivotal tools in addressing intricate bacterial infections. Nonetheless, the rise of resistance to these crucial last-resort antibiotics constitutes a substantial and pressing threat to the overall well-being of the global population.
Limitations
This scoping review is subject to certain limitations, the foremost being the absence of CD4 count data in a substantial number of studies, thereby impeding a comprehensive assessment of immune status. Moreover, the methodological divergence among various study designs presents challenges in making direct comparisons. The predominance of studies conducted in African nations may constrain the extrapolation of findings to other geographical regions. Furthermore, the restricted reporting of resistance mechanisms and variations in specimen types and reporting criteria introduce complexities in data synthesis. In addition, there is a possibility of publication bias, favoring studies with statistically significant results. Moreover, the exclusion of non-English articles potentially introduces a language bias as relevant non-English publications may have been omitted.
Conclusion
A noteworthy discovery arising from this review is the uniformity observed in carbapenem resistance patterns between patients infected with HIV and those without the infection. This implies that HIV status may not serve as a prominent factor influencing carbapenem resistance within these patient cohorts. Furthermore, this review underscores the utmost significance of remaining vigilant and actively pursuing research endeavors in light of the escalating challenge posed by carbapenem resistance. The results suggest the necessity for adopting multifaceted strategies, encompassing judicious antibiotic utilization, stringent infection control protocols, and the innovation of novel therapeutic approaches to effectively combat the burgeoning menace of antibiotic resistance within healthcare settings.
Authors’ Contribution
Conceptualization: Kylie Divashnee Konar, Rushern Ruvashin Chetty, Selina Konar.
Data curation: Kylie Divashnee Konar, Rushern Ruvashin Chetty, Selina Konar.
Formal analysis: Kylie Divashnee Konar.
Investigation: Kylie Divashnee Konar, Rushern Ruvashin Chetty, Selina Konar.
Methodology: Kylie Divashnee Konar, Rushern Ruvashin Chetty, Selina Konar, Somasundram Pillay.
Project administration: Kylie Divashnee Konar, Rushern Ruvashin Chetty, Selina Konar, Somasundram Pillay.
Resources: Kylie Divashnee Konar, Rushern Ruvashin Chetty.
Supervision: Somasundram Pillay.
Validation: Somasundram Pillay.
Visualization: Kylie Divashnee Konar, Rushern Ruvashin Chetty, Selina Konar, Somasundram Pillay.
Writing–original draft: Kylie Divashnee Konar, Rushern Ruvashin Chetty, Selina Konar, Somasundram Pillay.
Writing–review & editing: Kylie Divashnee Konar, Rushern Ruvashin Chetty, Selina Konar, Somasundram Pillay.
Competing Interests
None.
Ethical Approval
Not applicable.
Funding
None.
References
- World Health Organization (WHO). Antibiotic Resistance. https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance. Accessed September 1, 2023.
- Shallcross L, Beckley N, Rait G, Hayward A, Petersen I. Antibiotic prescribing frequency amongst patients in primary care: a cohort study using electronic health records. J Antimicrob Chemother 2017; 72(6):1818-24. doi: 10.1093/jac/dkx048 [Crossref] [ Google Scholar]
- Llor C, Bjerrum L. Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Ther Adv Drug Saf 2014; 5(6):229-41. doi: 10.1177/2042098614554919 [Crossref] [ Google Scholar]
- Mancuso G, Midiri A, Gerace E, Biondo C. Bacterial antibiotic resistance: the most critical pathogens. Pathogens 2021; 10(10):1310. doi: 10.3390/pathogens10101310 [Crossref] [ Google Scholar]
- Santajit S, Indrawattana N. Mechanisms of antimicrobial resistance in ESKAPE pathogens. Biomed Res Int 2016; 2016:2475067. doi: 10.1155/2016/2475067 [Crossref] [ Google Scholar]
- Ventola CL. The antibiotic resistance crisis: part 1: causes and threats. P T 2015; 40(4):277-83. [ Google Scholar]
- Papp-Wallace KM, Endimiani A, Taracila MA, Bonomo RA. Carbapenems: past, present, and future. Antimicrob Agents Chemother 2011; 55(11):4943-60. doi: 10.1128/aac.00296-11 [Crossref] [ Google Scholar]
- Aurilio C, Sansone P, Barbarisi M, Pota V, Giaccari LG, Coppolino F. Mechanisms of action of carbapenem resistance. Antibiotics (Basel) 2022; 11(3):421. doi: 10.3390/antibiotics11030421 [Crossref] [ Google Scholar]
- Rima M, Oueslati S, Dabos L, Daaboul D, Mallat H, Bou Raad E. Prevalence and molecular mechanisms of carbapenem resistance among gram-negative bacilli in three hospitals of northern Lebanon. Antibiotics (Basel) 2022; 11(10):1295. doi: 10.3390/antibiotics11101295 [Crossref] [ Google Scholar]
- Wielders CCH, Schouls LM, Woudt SHS, Notermans DW, Hendrickx APA, Bakker J. Epidemiology of carbapenem-resistant and carbapenemase-producing Enterobacterales in the Netherlands 2017-2019. Antimicrob Resist Infect Control 2022; 11(1):57. doi: 10.1186/s13756-022-01097-9 [Crossref] [ Google Scholar]
- Zuma K, Simbayi L, Zungu N, Moyo S, Marinda E, Jooste S. The HIV epidemic in South Africa: key findings from 2017 national population-based survey. Int J Environ Res Public Health 2022; 19(13):8125. doi: 10.3390/ijerph19138125 [Crossref] [ Google Scholar]
- El-Atrouni W, Berbari E, Temesgen Z. HIV-associated opportunistic infections Bacterial infections. J Med Liban 2006; 54(2):80-3. [ Google Scholar]
- Habboush Y, Guzman N. Antibiotic resistance. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK513277/. Updated June 20, 2023.
- Peters MD, Marnie C, Tricco AC, Pollock D, Munn Z, Alexander L. Updated methodological guidance for the conduct of scoping reviews. JBI Evid Synth 2020; 18(10):2119-26. doi: 10.11124/jbies-20-00167 [Crossref] [ Google Scholar]
- Westphaln KK, Regoeczi W, Masotya M, Vazquez-Westphaln B, Lounsbury K, McDavid L. From Arksey and O’Malley and Beyond: customizations to enhance a team-based, mixed approach to scoping review methodology. MethodsX 2021; 8:101375. doi: 10.1016/j.mex.2021.101375 [Crossref] [ Google Scholar]
- Omulo S, Ita T, Mugoh R, Ayodo C, Luvsansharav U, Bollinger S. Risk factors for colonization with extended-spectrum cephalosporin-resistant and carbapenem-resistant Enterobacterales among hospitalized patients in Kenya: an antibiotic resistance in communities and hospitals (ARCH) study. Clin Infect Dis 2023; 77(Suppl 1):S97-103. doi: 10.1093/cid/ciad258 [Crossref] [ Google Scholar]
- Singh R, Mewa Kinoo S, Ramjathan P, Swe Swe-Han K, Singh B. Microbiological analysis and predictors of gallbladder infection with antimicrobial susceptibility patterns in an HIV setting. S Afr Med J 2023; 113(6):57-63. doi: 10.7196/SAMJ.2023.v113i6.442 [Crossref] [ Google Scholar]
- Lowe M, Shuping L, Perovic O. Carbapenem-resistant Enterobacterales in patients with bacteraemia at tertiary academic hospitals in South Africa, 2019 - 2020: an update. S Afr Med J 2022; 112(8):542-52. doi: 10.7196/SAMJ.2022.v112i8.16351 [Crossref] [ Google Scholar]
- Abongomera G, Koller M, Musaazi J, Lamorde M, Kaelin M, Tasimwa HB. Spectrum of antibiotic resistance in UTI caused by Escherichia coli among HIV-infected patients in Uganda: a cross-sectional study. BMC Infect Dis 2021; 21(1):1179. doi: 10.1186/s12879-021-06865-3 [Crossref] [ Google Scholar]
- Rameshkumar MR, Arunagirinathan N, Senthamilselvan B, Swathirajan CR, Solomon SS, Vignesh R. Occurrence of extended-spectrum β-lactamase, AmpC, and carbapenemase-producing genes in gram-negative bacterial isolates from human immunodeficiency virus infected patients. J Infect Public Health 2021; 14(12):1881-6. doi: 10.1016/j.jiph.2021.11.008 [Crossref] [ Google Scholar]
- Tessema NN, Ali MM, Zenebe MH. Bacterial associated urinary tract infection, risk factors, and drug susceptibility profile among adult people living with HIV at Haswassa University Comprehensive Specialized Hospital, Hawassa, Southern Esthiopia. Sci Rep 2020; 10(1):10790. doi: 10.1038/s41598-020-67840-7 [Crossref] [ Google Scholar]
- Ngalani OJT, Mbaveng AT, Marbou WJT, Ngai RY, Kuete V. Antibiotic resistance of enteric bacteria in HIV-infected patients at the Banka Ad-Lucem Hospital, west region of Cameroon. Can J Infect Dis Med Microbiol 2019; 2019:9381836. doi: 10.1155/2019/9381836 [Crossref] [ Google Scholar]
- Swathirajan CR, Rameshkumar MR, Solomon SS, Vignesh R, Balakrishnan P. Changing drug resistance profile in Pseudomonas aeruginosa infection among HIV patients from 2010-2017: a retrospective study. J Glob Antimicrob Resist 2019; 16:274-7. doi: 10.1016/j.jgar.2018.10.019 [Crossref] [ Google Scholar]
- Yang J, Tang Q, Qi T, Chen J, Ji Y, Tang Y. Characteristics and Outcomes of Acinetobacter baumannii infections in patients with HIV: a matched case-control study. Sci Rep 2018; 8(1):15617. doi: 10.1038/s41598-018-33753-9 [Crossref] [ Google Scholar]
- Reinheimer C, Keppler OT, Stephan C, Wichelhaus TA, Friedrichs I, Kempf VA. Elevated prevalence of multidrug-resistant gram-negative organisms in HIV positive men. BMC Infect Dis 2017; 17(1):206. doi: 10.1186/s12879-017-2286-z [Crossref] [ Google Scholar]
- Chiou CS, Izumiya H, Kawamura M, Liao YS, Su YS, Wu HH, et al. The worldwide spread of ciprofloxacin-resistant Shigella sonnei among HIV-infected men who have sex with men, Taiwan. Clin Microbiol Infect 2016;22(4):383.e11-383.e16. 10.1016/j.cmi.2015.12.021.
- Luyt CE, Aubry A, Lu Q, Micaelo M, Bréchot N, Brossier F. Imipenem, meropenem, or doripenem to treat patients with Pseudomonas aeruginosa ventilator-associated pneumonia. Antimicrob Agents Chemother 2014; 58(3):1372-80. doi: 10.1128/aac.02109-13 [Crossref] [ Google Scholar]
- Iweriebor BC, Obi CL, Akinyemi O, Ramalivhana NJ, Hattori T, Okoh AI. Uropathogens isolated from HIV-infected patients from Limpopo province, South Africa. Afr J Biotechnol 2012; 11(46):10598-604. doi: 10.5897/ajb10.2413 [Crossref] [ Google Scholar]
- Samie A, Shivambu N. Biofilm production and antibiotic susceptibility profiles of Staphylococcus aureus isolated from HIV and AIDS patients in the Limpopo province, South Africa. Afr J Biotechnol 2011; 10(65):14625-36. doi: 10.5897/ajb11.1287 [Crossref] [ Google Scholar]
- Srifuengfung S, Tribuddharat C, Yungyuen T, Wensentia T. Respiratory tract infection caused by bacteria (non-Mycobacterium) and their antibiogram in HIV-positive patients. Southeast Asian J Trop Med Public Health 2005; 36(3):709-12. [ Google Scholar]
- Center for Strategic and International Studies. The World’s Largest HIV Epidemic in Crisis: HIV in South Africa. Available from: https://www.csis.org/analysis/worlds-largest-hiv-epidemic-crisis-hiv-south-africa. Accessed September 28, 2022.
- Statista. Wealth in Africa by Country. Available from: https://www.statista.com/statistics/1182815/wealth-in-africa-by-country/. Accessed September 28, 2023.
- Doron S, Gorbach SL. Bacterial infections: overview. In: International Encyclopedia of Public Health. Elsevier; 2008. p. 273-82. 10.1016/b978-012373960-5.00596-7.
- Lin Q, Wang Y, Yu J, Li S, Zhang Y, Wang H. Bacterial characteristics of carbapenem-resistant Enterobacteriaceae (CRE) colonized strains and their correlation with subsequent infection. BMC Infect Dis 2021; 21(1):638. doi: 10.1186/s12879-021-06315-0 [Crossref] [ Google Scholar]
- Sheu CC, Chang YT, Lin SY, Chen YH, Hsueh PR. Infections caused by carbapenem-resistant Enterobacteriaceae: an update on therapeutic options. Front Microbiol 2019; 10:80. doi: 10.3389/fmicb.2019.00080 [Crossref] [ Google Scholar]
- Stirrup OT, Sabin CA, Phillips AN, Williams I, Churchill D, Tostevin A. Associations between baseline characteristics, CD4 cell count response and virological failure on first-line efavirenz + tenofovir + emtricitabine for HIV. J Virus Erad 2019; 5(4):204-11. [ Google Scholar]
- Liu P, You Y, Liao L, Feng Y, Shao Y, Xing H. Impact of low-level viremia with drug resistance on CD4 cell counts among people living with HIV on antiretroviral treatment in China. BMC Infect Dis 2022; 22(1):426. doi: 10.1186/s12879-022-07417-z [Crossref] [ Google Scholar]
- Poirel L, Madec JY, Lupo A, Schink AK, Kieffer N, Nordmann P, et al. Antimicrobial resistance in Escherichia coli. Microbiol Spectr 2018;6(4). 10.1128/microbiolspec.ARBA-0026-2017.
- Murugan MS, Sinha DK, Vinodh Kumar OR, Yadav AK, Pruthvishree BS, Vadhana P. Epidemiology of carbapenem-resistant Escherichia coli and first report of blaVIM carbapenemases gene in calves from India. Epidemiol Infect 2019; 147:e159. doi: 10.1017/s0950268819000463 [Crossref] [ Google Scholar]
- Piperaki ET, Tzouvelekis LS, Miriagou V, Daikos GL. Carbapenem-resistant Acinetobacter baumannii: in pursuit of an effective treatment. Clin Microbiol Infect 2019; 25(8):951-7. doi: 10.1016/j.cmi.2019.03.014 [Crossref] [ Google Scholar]