Avicenna Journal of Clinical Microbiology and Infection. 11(1):22-27.
doi: 10.34172/ajcmi.3474
Original Article
Evaluation of Antimicrobial Susceptibility in Extended-Spectrum β-Lactamase Producing Escherichia coli Isolates From Patients in Northwest Iran
Mohammad Bahloli 1  , Hossein Samadi Kafil 2, Parisa Roshani Asl 3, Niloufar Rashidi 3, *
, Hossein Samadi Kafil 2, Parisa Roshani Asl 3, Niloufar Rashidi 3, *  , Seyyed Reza Moaddab 4, *
, Seyyed Reza Moaddab 4, * 
Author information:
1Department of Hematology, Faculty of Allied Medicine, Iran University of Medical Sciences, Tehran, Iran
2Department of Microbiology, Faculty Medicine, Tabriz University of Medical Science, Tabriz, Iran
3Department of Medical Laboratory Sciences, Faculty of Allied Medicine, Iran University of Medical Sciences, Tehran, Iran
4Department of Medical Laboratory Sciences, Faculty of Allied Medicine, Tabriz University of Medical Science, Tabriz, Iran
Abstract
Background: Escherichia coli is one of the main causes of various diseases worldwide, whose multidrug-resistant strains have caused many public health problems by producing extended-spectrum β-lactamases (ESBLs). The resistance rate varies in different regions. Thus, it is necessary to identify ESBL-producing strains in each region and their antibiotic sensitivity in order to find appropriate treatment options. Hence, the present study aimed to detect the ESBL-producing E. coli strains and their antimicrobial susceptibility pattern in Tabriz, Iran.
Methods: This study was conducted at the Imam Reza Hospital in Tabriz from November 20, 2022, to April 20, 2023. A total of 400 E. coli isolates were collected from different clinical specimens. Antimicrobial susceptibility testing was performed by the disk diffusion method. ESBL-producing isolates were detected by the double-disc synergy test method according to the Clinical and Laboratory Standards Institute (CLSI) guidelines.
Results: Out of 400 E. coli isolates, 211 (52.75%) were obtained from females, and 189 (47.25%) belonged to males. The mean age of patients was 52.1±27.9 years. Overall, 279 (69.75%) were confirmed as ESBL producers. These producers were mainly recovered from outpatients. The highest antibiotic resistance was observed to ceftriaxone (86.25%) and tetracycline (80.75%), and the least antibiotic resistance was related to imipenem (8%) and amikacin (16.25%), respectively. The rate of antibiotic resistance among ESBL producers was higher than among non-ESBLs.
Conclusion: The present study reported a high prevalence of ESBL-producing E. coli among patients referring to Imam Reza hospital in Tabriz. Carbapenems, aminoglycosides, and nitrofurantoins were confirmed as the most efficient drugs for these bacteria, whereas cephalosporins, fluoroquinolones, and sulfonamides were the least effective agents.
Keywords: Escherichia coli, Antibiotics, Antimicrobial susceptibility, Extended-spectrum β-lactamase
Copyright and License Information
© 2024 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Please cite this article as follows: Bahloli M, Samadi Kafil H, Roshani Asl P, Rashidi N, Moaddab SR. Evaluation of Antimicrobial Susceptibility in Extended-Spectrum β-Lactamase Producing Escherichia coli Isolates From Patients in Northwest Iran. Avicenna J Clin Microbiol Infect. 2024; 11(1):22-27. doi:10.34172/ajcmi.3474
Introduction
Today, despite numerous advances in healthcare, the treatment of infections caused by drug-resistant bacteria remains a significant threat to public health (1). The indiscriminate use of antibiotics is identified as a primary factor in the development and spread of antimicrobial resistance among bacterial species (2). Infections with Escherichia coli are characterized by the production of virulence factors, including toxins that disrupt the intestinal mucosa, leading to symptoms such as diarrhea, abdominal pain, and fever. The spectrum of E. coli infections ranges from mild gastroenteritis to more severe conditions such as urinary tract infections, bacteremia, and potentially fatal kidney failure (3).Currently, β-lactam antibiotics are the most commonly prescribed class of antibacterial agents in clinical practice due to their excellent safety and broad antimicrobial spectrum. Unfortunately, resistance to β-lactams is rapidly increasing (4). The production of β-lactamase enzymes is the most common mechanism of resistance to β-lactam antibiotics, catalyzing the hydrolysis of the amide bond in the β-lactam ring, rendering the antibiotics inactive by no longer binding to the transpeptidase target enzymes involved in cell wall biosynthesis (5). Extended-spectrum beta-lactamases (ESBLs) constitute a critical category of β-lactamase enzymes, encoded either chromosomally or on plasmids, conferring resistance to penicillins, cephalosporins, and monobactams (6).
ESBLs are predominantly produced by gram-negative bacilli, particularly Enterobacteriaceae (7). Among the various members of the Enterobacteriaceae family, ESBL-producing E. coli has been identified as one of the leading multidrug-resistant bacteria implicated in severe hospital and community-acquired infections worldwide. Diseases caused by these bacteria result in substantial medical costs and limit treatment options (8). The aim of this study is to highlight the growing threat of drug-resistant E. coli infections, particularly those involving ESBL-producing strains. It also emphasizes the impact of indiscriminate antibiotic use on the development and spread of antimicrobial resistance and the challenges posed by the increasing resistance to β-lactam antibiotics. Additionally, it underscores the significant clinical and economic burden imposed by ESBL-producing E. coli infections, particularly in healthcare settings and the community.
Materials and Methods
Study Population and Bacterial Isolates
This study involved 400 E. coli isolates obtained from various clinical samples, including urine, blood, wound, cerebrospinal fluid, respiratory fluid such as sputum., and fecal samples. These samples were collected from inpatients and outpatients at Imam Reza hospital in Tabriz, Iran, from November 2022 to April 2023 (Research ethical code: IR. TBZMED. REC.1398. 779). The E. coli isolates were identified and isolated using culture methods (MacConkey agar test; this test involves culturing the bacterial sample on MacConkey agar, a selective and differential media that allows for the growth of E. coli). The presence of lactose-fermenting colonies indicates the likely presence of E. coli. Biochemical tests include the indole test, methyl red test, Voges-Proskauer test, and citrate utilization test (9).
Antimicrobial Susceptibility Testing
The antibiotic sensitivity of the isolates was determined by the standard Kirby-Bauer disk diffusion method on Mueller-Hinton agar medium (Millipore, Billerica, MA, USA) according to CLSI guidelines. Paper disks containing specific antibiotics are placed on an agar plate inoculated with bacteria. After the 24-hour incubation, the zone of inhibition (where bacteria do not grow) around the disks is measured to determine the susceptibility of the bacteria to the antibiotics. This method provides a qualitative assessment of bacterial susceptibility to different antibiotics (9).
Eleven commercially available antibiotic disks (Mast, UK) were used, including gentamicin (10 μg), amikacin (30 μg), ciprofloxacin (5 μg), ceftazidime (30 μg), cefotaxime (30 μg), imipenem (10 μg), tetracycline (30 μg), ceftriaxone, nitrofurantoin (300 μg), nalidixic acid (30 μg), and co-trimoxazole (25 mg). E. coli ATCC 25922 and ATTCC 35218 MDR strains were used as negative and positive controls, respectively (10).
Detection of Extended-Spectrum Beta-Lactamase-Producing Isolates
All E. coli isolates were tested for detecting ESBL production by the double-disc synergy test method as stated by CLSI recommendations (9). In this method, after bacterial culture, antibiotic disks (Mast, UK) in pairs of ceftazidime (30 μg) with ceftazidime/clavulanic acid (30/10 μg) and cefotaxime (30 μg) with cefotaxime/clavulanic acid (30/10 μg) were placed on the Mueller–Hinton agar medium, at a distance of 20 mm apart from each other. The diameter of the inhibition zone was measured after 24 hours of incubation of the plates at 37 °C (Figure 1). Based on the report of the CLSI, an increase of ≥ 5 mm in the diameter of the inhibition zones around the clavulanic acid combination disks versus the single antibiotic disk confirmed the presence of ESBL-producing isolates.E. coli ATCC 25922 and ATTCC 35218 were utilized as negative and positive control strains, respectively.
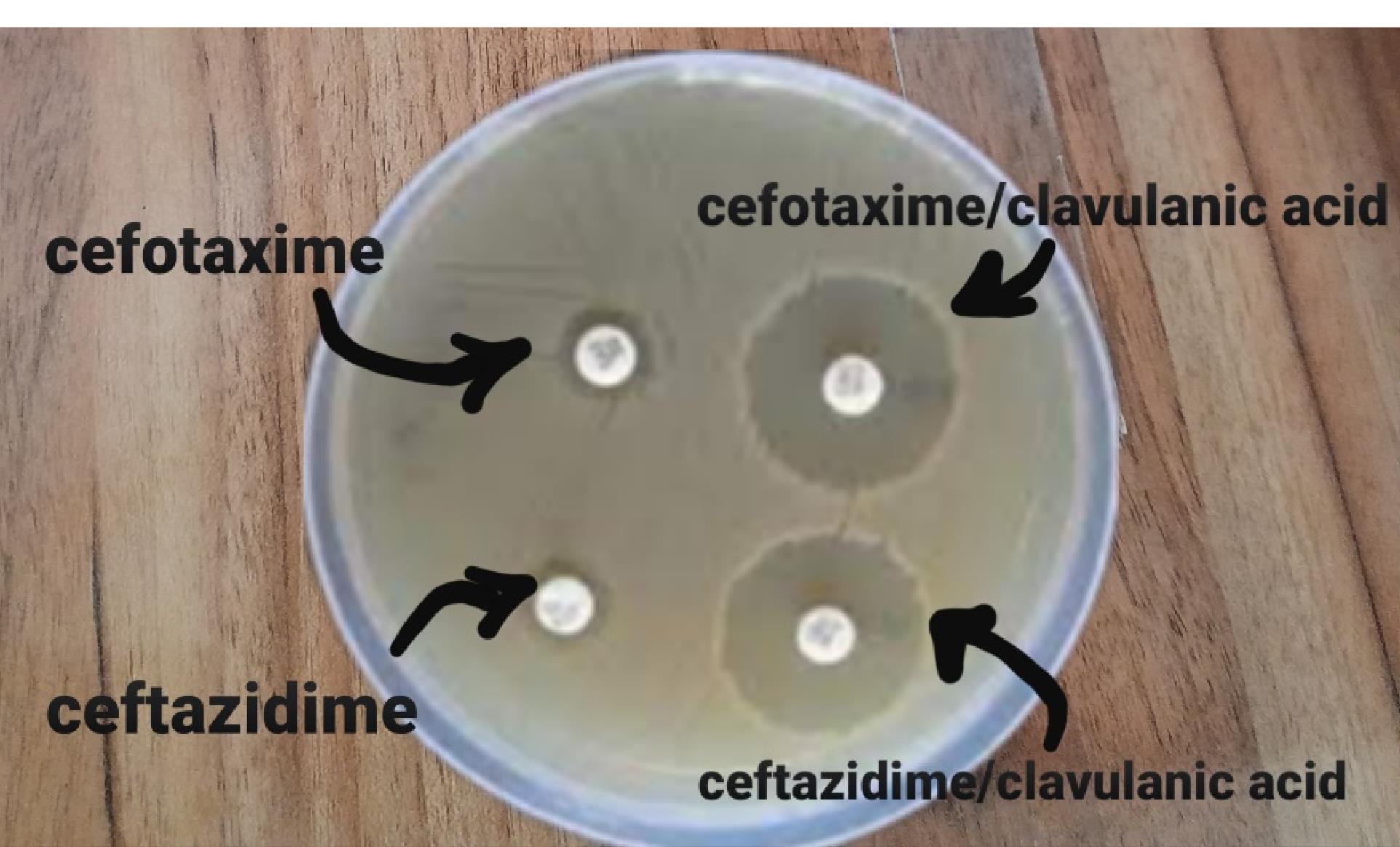
Figure 1.
Double Disc Difusion for ESBL Identificaction. Note. ESBL: Extended-spectrum β-lactamase
.
Double Disc Difusion for ESBL Identificaction. Note. ESBL: Extended-spectrum β-lactamase
Statistical Analysis
The expression ratio was measured using the Pfaffl formula, calculated manually (11). The data were analyzed by descriptive statistics, the chi-square statistical test, and Fisher’s exact test using SPSS, version 26 (IBM SPSS Statistics, New York, USA). The confidence limits for statistical tests were considered below 0.05.
Results
Demographic Information of the Participants
Out of the 400 E. coli isolates, 211 (52.75%) were recovered from females, and 189 (47.25%) were related to males. The isolates were primarily found in the urine (n = 287), followed by blood (n = 81) and wounds (n = 11). The patients’ mean age was 52.1 ± 27.9 years, with their ages ranging from 1 month to 80 years. As indicated in Table 1, the patients mostly belonged to the age groups of 60–80 years old (n = 226, 56%). The percentage of E. coli isolation among inpatients was 84%, while it was 15.75% in outpatients.
Table 1.
Demographic Data About the Source ofEscherichia coli Isolates and the Rate of ESBL-producing Strains
|
Variables
|
|
Total of 400
E. coli
Strains
|
%Among 400 Strains
|
ESBLs+Strains n (%)
|
| Gender (Inpatients and outpatients) |
Male |
189 |
47.25 |
121 (64) |
| Female |
211 |
52.75 |
158 (74.8) |
| Total |
Male and Female |
400 |
100 |
279 (69.75) |
| Age |
< 10 |
6 |
1.5 |
5 (83.3) |
| 10-20 |
13 |
3.25 |
8 (61.5) |
| 20-40 |
44 |
11 |
32 (72.2) |
| 40-60 |
111 |
27.75 |
85 (76.5) |
| 60-80 |
226 |
56.5 |
149 (65) |
| Total |
1-80 |
400 |
100 |
279 (69.75) |
| Samples type |
Urine |
287 |
71.75 |
198 (69) |
| Blood |
81 |
20.25 |
64 (79.2) |
| Wound swab |
11 |
2.75 |
7 (63.6) |
| Others (wound, CSF, saliva, and excrement) |
17 |
4.25 |
10 (58.8) |
| Total |
|
400 |
100 |
279 (69.75) |
| Samples form |
|
|
|
|
| Outpatients |
|
63 |
15.75 |
51 (82.25) |
| Orology |
73 |
18.25 |
43 (59) |
| Infectious |
26 |
6.5 |
18 (69.2) |
| Pulmonary care |
22 |
5.5 |
19 (86.6) |
| Gastro enology |
19 |
4.75 |
18 (94.7) |
| Glandsand rheumatology |
18 |
4.5 |
12 (66.6) |
| General interior |
17 |
4.25 |
11 (64.7) |
| ICU nerves |
17 |
4.25 |
13 (76.4) |
| Inpatients |
ICU surgery unite |
17 |
4.25 |
13 (76.4) |
| ICU surgery |
15 |
3.75 |
14 (93.3) |
| ICU lung |
14 |
3.5 |
11 (57.1) |
| Brain |
13 |
3.25 |
8 (61.5) |
| ENT |
11 |
2.75 |
11 (100) |
| Stroke care unit |
11 |
2.75 |
3 (27.2) |
| Organ transplants |
10 |
2.5 |
8 (80) |
| Orthopedic |
7 |
1.75 |
3 (42.8) |
| ICU nerves |
5 |
1.25 |
4 (80) |
| Thorax surgery |
5 |
1.25 |
4 (80) |
Note. ICU: Intensive care unit; ENT: Ear, nose, and throat; ESBL: Extended-spectrum β-lactamase; CSF: Cerebrospinal fluid.
Antimicrobial Susceptibility Profile and ESBL Production
Table 2 reports the antimicrobial susceptibility pattern of all 400 E. coli isolates for tested antibiotics. The highest resistance rate belonged to ceftriaxone (86.25%), tetracycline (80.75%), ciprofloxacin (79.5%), and co-trimoxazole (78.5%), while the least resistance was related to imipenem (8%) and amikacin (16.25%).
Table 2.
Drug Resistance Rate Among 400 Escherichia coli Isolates n (%)
|
Drugs
|
Resistance Rate Among 279 ESBL-Producing
E.coli
Isolates n (%)
|
Resistance Rate Among 121
Non-ESBL
E. coli
Isolates n (%)
|
| Ceftriaxone |
92 |
70.2 |
| Tetracycline |
84.3 |
71.4 |
| Ciprofloxacin |
82.37 |
71.89 |
| Co-trimoxazol |
80.56 |
73.17 |
| Ceftazidim |
76.61 |
42.85 |
| Cefotaxime |
72 |
70.37 |
| Naldixic acid |
72 |
68 |
| Gentamycin |
49.27 |
37.78 |
| Nitrofurantoin |
21.13 |
16.14 |
| Amikacin |
18.2 |
11.89 |
| Imipenem |
9.1 |
6.03 |
Note. ESBL: Extended-spectrum β-lactamase.
Of the 400 E. coli isolates, 279 (69.75%) were identified as ESBL producers. The highest percentage of ESBL-producing isolates was detected in children with an age range above 10 (83.3%) (Figure 2). The most and least number of ESBL-positive isolates belonged to the outpatients (80.95%) and special care unit patients (27.2%), respectively. In terms of sample type, most ESBL producers were isolated from blood samples (79%), followed by those obtained from urine samples (68.9%) (Table 1). According to the information presented in Table 2, the expression of ESBLs resulted in the development of resistance to all antibiotics, especially ceftriaxone, tetracycline, ciprofloxacin, and co-trimoxazole.
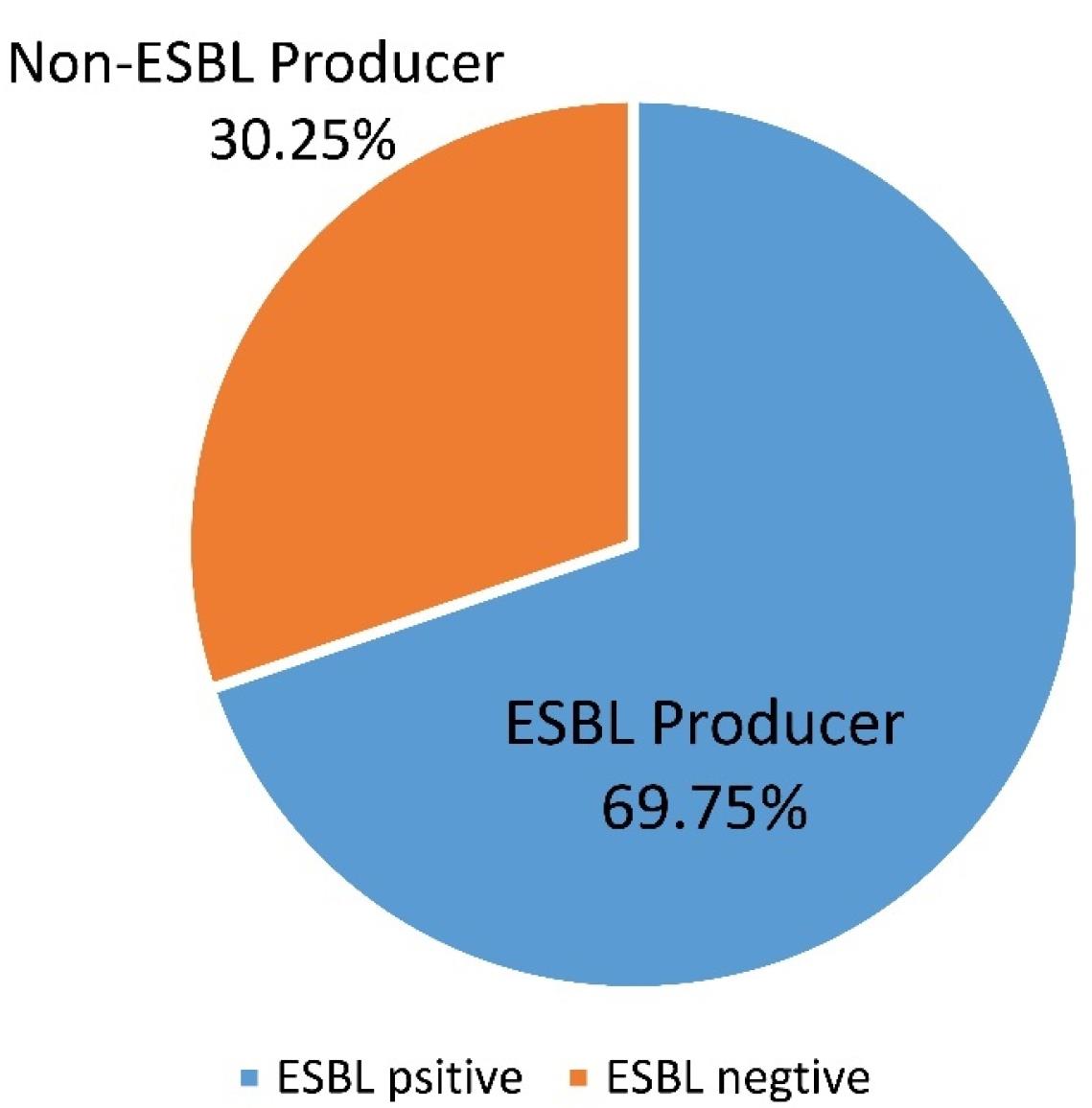
Figure 2.
Percentage of ESBL and Non-ESBL-producing Isolates. Note. ESBL: Extended-spectrum β-lactamase
.
Percentage of ESBL and Non-ESBL-producing Isolates. Note. ESBL: Extended-spectrum β-lactamase
Discussion
The emergence and rapid spread of multidrug resistance, particularly in ESBL-producing Enterobacteriaceae, pose a global health problem. These pathogens cause serious nosocomial and community infections, negatively affecting disease management (12). Understanding local epidemiology and the evolution of commonly isolated species, such as E. coli, is crucial for selecting effective treatments for each region.
The present study was conducted to determine the prevalence of ESBL-producing E. coli and its antimicrobial resistance profile based on the laboratory results of the patients attending the out-patient and inpatient departments of Imam Reza hospital in Tabriz, Iran, from November 2022 to April 2023 and to find an effective antibiotic strategy to prevent the spread of these strains. In this survey, 400 E. coli isolates were collected from different samples. The majority of the E. coli strains were isolated from urine samples (n = 287, 71.75%) of females (n = 211, 52.75%). This finding is in line with those of other studies reporting E. coli as the most common pathogen isolated from women with urinary tract infections compared to men (13,14). This could be due to women’s increased susceptibility to urinary tract infections because of anatomical factors and hormonal changes (15). It was found that the isolates were frequently related to ≥ 60-year-old patients. This is in conformity with the findings of other studies in which the outbreak of E. coli was higher among elderly patients (16,17).
In this study, 69.75% (n = 279/400) of the isolates were ESBL producers. In some previous studies conducted in different countries such as Iran, Turkey, and China, the prevalence of ESBL-producing E. coli has been reported as 89.8%, 84%, and 68.2%, respectively (18–20), which corroborates the results of our study. On the other hand, some other researchers reported the prevalence rate of ESBL-producing E. coli as 35.7% in Iran, 19.3% in Zimbabwe, and only 3% in India (21-23), which contradicts our findings. These differences can be due to the geographical area and the diagnostic methods used in the studies (24).
In the present study, the rate of ESBL-producing E. coli in outpatients was higher than in inpatients (80.95% vs. 53.25%). This is consistent with the results of other published studies, which revealed that over the recent decades, ESBL-producing enterobacteria, especially E. coli, have emerged as important pathogens in outpatients in many areas of the world (25-28). In determining the antibiotic susceptibility patterns of E. coli by commonly prescribed antibiotics, the highest resistance belonged to ceftriaxone (86.25%), tetracycline (80.75%), ciprofloxacin (79.5%), and co-trimoxazole (78.5%), while the least resistance was related to imipenem (8%), amikacin (16.25%), and nitrofurantoin (19.75%).
Further, this study indicated that ESBL-producing isolates had higher resistance to tested antibiotics compared to non-ESBL producers. Nevertheless, both groups of isolates were highly sensitive to imipenem and amikacin. Many studies around the world have provided similar results in this regard. For example, the study conducted by Rodriguez-Baño et al showed that resistance to imipenem and amikacin was 5.98% and 5.1%, respectively (29). In the study by Hashemizadeh et al, 96.2%, 85.1%, and 72.6% susceptibility to imipenem, amikacin, and nitrofurantoin were observed, respectively (30). In another study by Khan et al (31), the isolates were highly resistant to tetracycline (95%), ciprofloxacin (85%), ceftriaxone (88%), cefotaxime (78%), ceftazidime (63%), and gentamicin (60%), while they were highly susceptible to imipenem (91%). According to a report by Lautenbach et al, the resistance rates to nalidixic acid, ceftriaxone, gentamicin, amikacin, and nitrofurantoin were 84%, 73%, 36%, 12%, and 8%, respectively (32). In addition, Pourakbari et al indicated that ESBL-positive bacteria were highly resistant to cefotaxime (100%), ceftriaxone (100%), and ceftazidime (70.6%), while both ESBL-producing and non-ESBL-producing isolates demonstrated low resistance to amikacin (9.5%), and no resistance was observed toward imipenem (33). In line with the results of our study and these similar investigations, carbapenems (imipenem) and aminoglycosides (amikacin) are the best options to prescribe against both ESBL-producing E. coli and non-ESBL-producing isolates.
Limitations of the Study
The number of samples was limited due to high financial costs.
Conclusion
The findings of the current study demonstrated a high rate of infection with ESBL-producing E. coli isolates. The ESBL producers were primarily outpatients. High resistance to different types of antibiotics was observed in all isolates. The amount of resistance in ESBL producers was higher than in non-ESBLs. Meanwhile, the analysis of antibiogram results revealed that carbapenems and aminoglycosides are suitable options for the treatment of ESBL-producing strains, while cephalosporins, fluoroquinolones, and sulfonamides are not recommended and their prescription should be limited. Finally, due to the spread of these isolates in the community and increasing resistance to most of the common antibiotics, it is necessary to carefully screen the isolates before prescribing the drug to closely monitor antibiotic prescriptions and choose the appropriate and effective treatment options.
Acknowledgments
The authors are grateful to the research staff of the Department of Medical Laboratory Sciences, Faculty of Allied Medical Science, Tabriz University of Medical Sciences, Iran, for their contribution to improving the quality of this research.
Authors’ Contribution
Conceptualization: Mohammad Bahloli, Niloufar Rashidi, Seyyed Reza Moaddab.
Data curation: Mohammad Bahloli, Hossein Samadi Kafil.
Formal analysis: Mohammad Bahloli, Parisa Roshani Asl.
Funding acquisition: Niloufar Rashidi, Seyyed Reza Moaddab.
Investigation: Mohammad Bahloli, Niloufar Rashidi.
Methodology: Mohammad Bahloli, Parisa Roshani Asl, Niloufar Rashidi, Seyyed Reza Moaddab.
Project administration: Niloufar Rashidi, Seyyed Reza Moaddab.
Resources: Mohammad Bahloli, Niloufar Rashidi, Seyyed Reza Moaddab.
Software: Mohammad Bahloli, Niloufar Rashidi, Seyyed Reza Moaddab.
Supervision: Niloufar Rashidi, Seyyed Reza Moaddab.
Validation: Niloufar Rashidi, Seyyed Reza Moaddab.
Visualization: Niloufar Rashidi, Seyyed Reza Moaddab.
Writing–original draft: Mohammad Bahloli, Niloufar Rashidi, Seyyed Reza Moaddab.
Writing–review & editing: Mohammad Bahloli, Niloufar Rashidi, Seyyed Reza Moaddab, Parisa Roshani Asl.
Competing Interests
The author declared no conflict of interests.
Ethical Approval
All ethical aspects of this research were approved by the Research and Ethics Committee of Tabriz University of Medical Sciences. The patients’ demographic data were collected from the medical records database, and their information remained confidential. Informed consent was obtained from all participants or their legal guardians before the study.
Funding
There is no funding or support for the publication of this study.
References
- Moaddab SR, Amini K, Kazemi Haki B. Determining the drug susceptibility of Mycobacterium tuberculosis strains to the pyrazinamide. Med J Tabriz Univ Med Sci Health Serv 2015;37(3):56-62. [Persian].
- Mancuso G, Midiri A, Gerace E, Biondo C. Bacterial antibiotic resistance: the most critical pathogens. Pathogens 2021; 10(10):1310. doi: 10.3390/pathogens10101310 [Crossref] [ Google Scholar]
- Peng Z, Wang X, Huang J, Li B. Pathogenic Escherichia coli. In: Tang YW, Hindiyeh MY, Liu D, Sails A, Spearman P, Zhang JR, eds. Molecular Medical Microbiology. Academic Press; 2024. p. 1065-96. 10.1016/b978-0-12-818619-0.00069-1.
- Ur Rahman S, Ali T, Ali I, Khan NA, Han B, Gao J. The growing genetic and functional diversity of extended spectrum beta-lactamases. Biomed Res Int 2018; 2018:9519718. doi: 10.1155/2018/9519718 [Crossref] [ Google Scholar]
- Patel MP, Hu L, Brown CA, Sun Z, Adamski CJ, Stojanoski V. Synergistic effects of functionally distinct substitutions in β-lactamase variants shed light on the evolution of bacterial drug resistance. J Biol Chem 2018; 293(46):17971-84. doi: 10.1074/jbc.RA118.003792 [Crossref] [ Google Scholar]
- Mofolorunsho KC, Ocheni HO, Aminu RF, Omatola CA, Olowonibi OO. Prevalence and antimicrobial susceptibility of extended-spectrum beta lactamases-producing Escherichia coli and Klebsiella pneumoniae isolated in selected hospitals of Anyigba, Nigeria. Afr Health Sci 2021; 21(2):505-12. doi: 10.4314/ahs.v21i2.4 [Crossref] [ Google Scholar]
- Martínez-Vázquez AV, Mandujano A, Cruz-Gonzalez E, Guerrero A, Vazquez J, Cruz-Pulido WL. Evaluation of retail meat as a source of ESBL Escherichia coli in Tamaulipas, Mexico. Antibiotics (Basel) 2022; 11(12):1795. doi: 10.3390/antibiotics11121795 [Crossref] [ Google Scholar]
- Mahmud ZH, Kabir MH, Ali S, Moniruzzaman M, Imran KM, Nafiz TN. Extended-spectrum beta-lactamase-producing Escherichia coli in drinking water samples from a forcibly displaced, densely populated community setting in Bangladesh. Front Public Health 2020; 8:228. doi: 10.3389/fpubh.2020.00228 [Crossref] [ Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing. 30th ed. CLSI Supplement M100. Wayne, PA: CLSI; 2020.
- Amoroso AM, Gutkind GO. Chromogenic detection of aminoglycoside phosphotransferases. Antimicrob Agents Chemother 1998; 42(2):228-30. doi: 10.1128/aac.42.2.228 [Crossref] [ Google Scholar]
- Nallapareddy SR, Murray BE. Ligand-signaled upregulation of Enterococcus faecalis ace transcription, a mechanism for modulating host-E faecalis interaction. Infect Immun 2006; 74(9):4982-9. doi: 10.1128/iai.00476-06 [Crossref] [ Google Scholar]
- Kettani Halabi M, Lahlou FA, Diawara I, El Adouzi Y, Marnaoui R, Benmessaoud R. Antibiotic resistance pattern of extended spectrum beta lactamase producing Escherichia coli isolated from patients with urinary tract infection in Morocco. Front Cell Infect Microbiol 2021; 11:720701. doi: 10.3389/fcimb.2021.720701 [Crossref] [ Google Scholar]
- Shakya P, Shrestha D, Maharjan E, Sharma VK, Paudyal R. ESBL production among E coli and Klebsiella spp causing urinary tract infection: a hospital-based study. Open Microbiol J 2017; 11:23-30. doi: 10.2174/1874285801711010023 [Crossref] [ Google Scholar]
- John AS, Mboto CI, Agbo B. A review on the prevalence and predisposing factors responsible for urinary tract infection among adults. Eur J Exp Biol 2016; 6(4):7-11. [ Google Scholar]
- Koren J, Hubenakova Z, Drahovska H, Ozaee E, Markuskova B, Lichvarikova A. Emergence of extended-spectrum β-lactamase (ESBL) and/or carbapenemase producing Enterobacteriaceae (CPE) and their antimicrobial resistance. Bratisl Lek Listy 2019; 120(12):935-40. doi: 10.4149/bll_2019_157 [Crossref] [ Google Scholar]
- Raja NS. Oral treatment options for patients with urinary tract infections caused by extended spectrum βeta-lactamase (ESBL) producing Enterobacteriaceae. J Infect Public Health 2019; 12(6):843-6. doi: 10.1016/j.jiph.2019.05.012 [Crossref] [ Google Scholar]
- Jabalameli L, Beigverdi R, Hagh Ranjbar H, Pouriran R, Jabalameli F, Emaneini M. Phenotypic and genotypic prevalence of extended-spectrum β-lactamase-producing Escherichia coli: a systematic review and meta-analysis in Iran. Microb Drug Resist 2021; 27(1):73-86. doi: 10.1089/mdr.2019.0396 [Crossref] [ Google Scholar]
- Burcu Bali E, Açık L, Sultan N. Phenotypic and molecular characterization of SHV, TEM, CTX-M and extended-spectrum beta-lactamase produced by Escherichia coli, Acinobacterbaumanniiand Klebsiella isolates in a Turkish hospital. Afr J Microbiol Res 2010; 4(8):650-4. [ Google Scholar]
- Liu H, Wang Y, Wang G, Xing Q, Shao L, Dong X. The prevalence of Escherichia coli strains with extended spectrum beta-lactamases isolated in China. Front Microbiol 2015; 6:335. doi: 10.3389/fmicb.2015.00335 [Crossref] [ Google Scholar]
- Gharavi MJ, Zarei J, Roshani-Asl P, Yazdanyar Z, Sharif M, Rashidi N. Comprehensive study of antimicrobial susceptibility pattern and extended spectrum beta-lactamase (ESBL) prevalence in bacteria isolated from urine samples. Sci Rep 2021; 11(1):578. doi: 10.1038/s41598-020-79791-0 [Crossref] [ Google Scholar]
- Olaru ID, Ferrand RA, Chisenga M, Yeung S, Macrae B, Chonzi P. Prevalence of ESBL-producing Escherichia coli in adults with and without HIV presenting with urinary tract infections to primary care clinics in Zimbabwe. JAC Antimicrob Resist 2021; 3(2):dlab082. doi: 10.1093/jacamr/dlab082 [Crossref] [ Google Scholar]
- Chen HE, Tain YL, Kuo HC, Hsu CN. Trends in antimicrobial susceptibility of Escherichia coli Isolates in a Taiwanese child cohort with urinary tract infections between 2004 and 2018. Antibiotics (Basel) 2020; 9(8):501. doi: 10.3390/antibiotics9080501 [Crossref] [ Google Scholar]
- Singh AK, Das S, Singh S, Gajamer VR, Pradhan N, Lepcha YD. Prevalence of antibiotic resistance in commensal Escherichia coli among the children in rural hill communities of Northeast India. PLoS One 2018; 13(6):e0199179. doi: 10.1371/journal.pone.0199179 [Crossref] [ Google Scholar]
- Gunjal PN, Gunjal SP. Determination of ESBL production & antibiotic resistance profile of Escherichia coli isolated from patients with urinary tract infections: a study from tertiary care hospital. VIMS Health Sci J 2020; 7(1):23-9. doi: 10.46858/vimshsj.7106 [Crossref] [ Google Scholar]
- Pitout JD, Nordmann P, Laupland KB, Poirel L. Emergence of Enterobacteriaceae producing extended-spectrum beta-lactamases (ESBLs) in the community. J Antimicrob Chemother 2005; 56(1):52-9. doi: 10.1093/jac/dki166 [Crossref] [ Google Scholar]
- Riyahi Zaniani F, Meshkat Z, Naderi Nasab M, Khaje-Karamadini M, Ghazvini K, Rezaee A. The prevalence of TEM and SHV genes among extended-spectrum beta-lactamases producing Escherichia coli and Klebsiella pneumoniae. Iran J Basic Med Sci 2012; 15(1):654-60. [ Google Scholar]
- Woodford N, Ward ME, Kaufmann ME, Turton J, Fagan EJ, James D. Community and hospital spread of Escherichia coli producing CTX-M extended-spectrum beta-lactamases in the UK. J Antimicrob Chemother 2004; 54(4):735-43. doi: 10.1093/jac/dkh424 [Crossref] [ Google Scholar]
- Rodriguez-Baño J, Paterson DL. A change in the epidemiology of infections due to extended-spectrum beta-lactamase-producing organisms. Clin Infect Dis 2006; 42(7):935-7. doi: 10.1086/500945 [Crossref] [ Google Scholar]
- Rodríguez-Baño J, Navarro MD. Extended-spectrum beta-lactamases in ambulatory care: a clinical perspective. Clin Microbiol Infect 2008; 14 Suppl 1:104-10. doi: 10.1111/j.1469-0691.2007.01866.x [Crossref] [ Google Scholar]
- Hashemizadeh Z, Kalantar-Neyestanaki D, Mansouri S. Clonal relationships, antimicrobial susceptibilities, and molecular characterization of extended-spectrum beta-lactamase-producing Escherichia coli isolates from urinary tract infections and fecal samples in Southeast Iran. Rev Soc Bras Med Trop 2018; 51(1):44-51. doi: 10.1590/0037-8682-0080-2017 [Crossref] [ Google Scholar]
- Khan E, Ejaz M, Zafar A, Jabeen K, Shakoor S, Inayat R. Increased isolation of ESBL producing Klebsiella pneumoniae with emergence of carbapenem resistant isolates in Pakistan: report from a tertiary care hospital. J Pak Med Assoc 2010; 60(3):186-90. [ Google Scholar]
- Lautenbach E, Patel JB, Bilker WB, Edelstein PH, Fishman NO. Extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae: risk factors for infection and impact of resistance on outcomes. Clin Infect Dis 2001; 32(8):1162-71. doi: 10.1086/319757 [Crossref] [ Google Scholar]
- Pourakbari B, Mamishi S, Shokrollahi MR, Heydari H, Mahmoudi S, Banar M. Molecular characteristics and antibiotic resistance profiles of Escherichia coli strains isolated from urinary tract infections in children admitted to children’s referral hospital of Qom, Iran. Ann Ig 2019; 31(3):252-62. doi: 10.7416/ai.2019.2288 [Crossref] [ Google Scholar]