Avicenna Journal of Clinical Microbiology and Infection. 9(4):157-164.
doi: 10.34172/ajcmi.2022.3416
Original Article
Decontamination of Salmonella Typhimurium and Listeria monocytogenes on Food-Related Surfaces by a Combination of Sodium Dodecyl Sulfate, Lactic Acid, or Citric Acid Under Different Temperatures
Siavash Maktabi 1, *  , Mehdi Pourmahdi Brojeni 1
, Mehdi Pourmahdi Brojeni 1  , Leila Elahinia 2
, Leila Elahinia 2
Author information:
1Department of Food Hygiene, Faculty of Veterinary Medicine, Shahid Chamran University of Ahvaz, Box: 61355-145, Ahvaz, Iran
2Graduated from Department of Food Hygiene, Faculty of Veterinary Medicine, Shahid Chamran University of Ahvaz, Iran
Abstract
Background:
Salmonella Typhimurium and Listeria monocytogenes are among the most important foodborne pathogens, and new methods to remove them from surfaces are useful. The aim of this study was to investigate the bactericidal effect of a combination of sodium dodecyl sulfate (SDS) and some food matrix-related factors such as temperature, salinity, acidity, and exposure time on L. monocytogenes and S. Typhimurium in suspension and on different food industry related surfaces.
Methods: The bacterial strains were treated with different concentrations of SDS, citric acid, lactic acid, and NaCl at different temperatures at various times. At least one concentration was selected that caused one or less log reduction in the viability of each bacterium, and the combination treatments were examined in this regard. The best combination was then selected, and its bactericidal effect on the bacteria tested was evaluated on ceramic, stainless steel, and plastic surfaces.
Results: The results showed that the sensitivity of the bacteria studied to different disinfectants was different. L. monocytogenes was highly sensitive to SDS, while S. Typhimurium was relatively resistant to SDS. Both bacteria were more sensitive to lactic acid than to citric acid, and the bactericidal effects of the disinfectants were enhanced in the combined treatments at 45°C compared to 35°C treatments. The addition of NaCl to the SDS solution resulted in a strong reduction in the bactericidal effect of SDS. The selected disinfectant removed bacterial biofilms from stainless steel surfaces in a shorter time than ceramic and plastic surfaces.
Conclusion: The preparation of combined solutions using SDS and an organic acid at an appropriate concentration and temperature could be useful for removing or reducing bacterial biofilms. Therefore, the combination of SDS and the lactic acid at 45°C can effectively remove pathogenic bacteria from various surfaces.
Keywords: Disinfectant, Food industry, Listeria monocytogenes, Salmonella Typhimurium, Sodium dodecyl sulfate
Copyright and License Information
© 2022 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Maktabi S, Pourmahdi Brojeni M, Elahinia L. Decontamination of Salmonella Typhimurium and listeria monocytogenes on food-related surfaces by a combination of sodium dodecyl sulfate, lactic acid, or citric acid under different temperatures. Avicenna J Clin Microbiol Infect. 2022; 9(4):157-164. doi:10.34172/ajcmi.2022.3416
Introduction
Salmonella Typhimuriumand Listeria monocytogenes, which have high prevalence and high mortality rates, are the main food-borne pathogens (1). Direct contact of food with contaminated food surfaces during preparation or packaging is a common route of food contamination. Conventional methods of cleaning food preparation surfaces are not always sufficient to remove food-borne pathogens, and it is, therefore, useful to use new methods that can be incorporated into food processing facilities (2).
Sodium dodecyl sulphate (SDS)is a highly effective anionic surfactant that has previously been used to disrupt membranes and denature proteins in bacteria (3). The US Food and Drug Administration has approved SDS as a generally recognized a safe multi-purpose food additive (4). The scientific community has considered the use of SDS as a disinfectant in food, equipment, and food contact surfaces (5,6).
Several researchers have studied the combination of SDS with other substances to improve their lethality. For example, the effect of a combination of 0.5% SDS and 5% lactic acid on the control of L. monocytogenes in vacuum-packed frankfurter sausages was studied for 90 days, and it was concluded that this combination could be an alternative way to control L. spp. in processed meat products (7). The effect of levulinic acid (LVA) and SDS solution on the control of S. Typhimurium and Escherichia coli O157 in lettuce and chicken skin was investigated in another study. The researchers reported that the combined solution is a good way to remove enteric pathogenic bacteria from fresh vegetables such as lettuce and chicken carcasses (8). Another study tested 3% LVA and 2% SDS solution as a foam to remove Salmonella spp. from cage walls and poultry carcasses and demonstrated a 4-log reduction in bacterial counts (9). The effects of different percentages of SDS, alone or in combination with other substances, on various foods such as ground beef (10), chicken breasts (11), raspberries (12), and eggshells (13) have been studied as well.
Although the synergistic effects of SDS with other materials have been documented, there is insufficient information on the effects of different environmental factors on the bactericidal effects of SDS. Therefore, this study aimed to investigate the synergistic effects of a combination of SDS and some food matrix-related factors such as temperature, salinity, acidity, and exposure time on L. monocytogenes and S. Typhimurium bacteria in a common brine solution and on various food industry-related surfaces.
Materials and Methods
Bacteria
Salmonella Typhimurium (ATCC35987) and L. monocytogenes (ATCC 7644) were obtained from the culture collection of the Department of Food Hygiene, Faculty of Veterinary Medicine, Shahid Chamran University of Ahvaz. All colonies were identified using biochemical tests and polymerase chain reaction (PCR) technique for confirmation.
Preparation of Bacterial Suspensions
To reach the maximum population of the studied bacteria for inoculation, a colony from a fresh agar plate culture was inoculated into 5 mL tryptic soy broth and incubated at 37°C for 20 hours. The cultivation was repeated twice, and then 100 μL aliquots of the bacterial suspensions were removed for viable counting.
Primary Treatments of Bacteria With Individual Parameters
To determine the sensitivity of each strain, an aliquot of the suspension of each bacterium was pipetted into different concentrations of SDS, citric acid, lactic acid, and NaCl solutions (Table 1) so that to obtain 1 × 106 CFU/mL of the bacterium and then treated for 0, 10, 20, 30, 40, and 60 minutes at different temperatures. Before and after each treatment, 10 μL volumes were taken for viable counts. Based on the sensitivity of each strain to the primary treatments, which produce approximately one log or less reduction in viability, several concentrations were chosen for combination treatments.
Table 1.
Concentration and Temperatures Used in Individual Treatments
|
Treatment
|
Concentration
|
| SDS (%) |
Different concentrations were used depending on the test conditions (from 0.009 to 3) |
| Citric acid (%) |
Different concentrations were used depending on the test conditions (from 0.05 to 0.2) |
| Lactic acid (%) |
Different concentrations were used depending on the test conditions (from 0.03 to 0.2) |
| NaCl (%) |
0 |
1 |
2 |
3 |
4 |
| Temperature (°C) |
4 |
25 |
35 |
45 |
55 |
Note. SDS: Sodium dodecyl sulfate.
Combined Treatment of Bacterial Suspension With Single, Two, and Three Parameters
Based on the selected concentrations from the previous step, the bacterial strains were treated in combination (Table 2) and the results were recorded. The best combination was then selected, and its bactericidal effect against the tested bacteria was evaluated on ceramic, stainless steel, and plastic surfaces.
Table 2.
Parameters Used on Salmonella Typhimurium and Listeria monocytogenes During Single and Combined Treatments
|
Factors
|
|
Range
|
|
Time: 0, 10, 20, 30, 40, and 60 minutes
|
|
Salmonella Typhimurium |
| Single-factor treatments |
SDS |
0.5%, 1%, 2%, 2.5%, & 3% |
| Citric acid |
0.03%, 0.1%, 0.15%, & 0.2% |
| Lactic acid |
0.02%, 0.07%, 0.1%, & 0.2% |
| NaCl |
1.5%, 2%, 3%, & 4% |
| Double-factor treatments |
SDS + Citric acid |
0.5% + 0.03%
2.5% + 0.1%
2.5% + 0.15% |
| SDS + Lactic acid |
0.5% + 0.02%
0.5% + 0.07% |
| SDS + Temperature |
0.5% + 4°C, 25°C, 35°C, 45°C, & 55°C |
| SDS + NaCl |
2.5% + 1.5%, 2%, 3%, & 4% |
| Three-factor treatments |
SDS + Citric acid + Temperature |
SDS 0.5% + Citric acid 0.03% + Temperature 45°C |
| SDS + Lactic acid + Temperature |
SDS 0.5% + Lactic acid 0.02% + Temperature 45°C |
|
Listeria monocytogenes
|
| Single-factor treatments |
SDS |
0.009%, 0.01%, 0.02%, & 0.03% |
| Citric acid |
0.05%, 0.3%, & 0.5% |
| Lactic acid |
0.03%, 0.1%, & 0.5% |
| NaCl |
1.5%, 2%, 3%, & 4% |
| Double-factor treatments |
SDS + Citric acid |
0.01% + 0.3%
0.009% + 0.05% |
| SDS + Lactic acid |
0.01% + 0.1%
0.009% + 0.03% |
| SDS + Temperature |
0.009% + 4°C, 25°C, 35°C, 45°C, & 55°C |
| SDS + NaCl |
0.01% + 1.5%, 2%, 3%, & 4% |
| Three-factor treatments |
SDS + Citric acid + Temperature |
SDS 0.009% + Citric acid 0.05% + Temperature 35°C |
| SDS + Lactic acid + Temperature |
SDS 0.009% + Lactic acid 0.03% + Temperature 35°C |
Note. SDS: Sodium dodecyl sulfate.
Preparation of Stainless Steel, Ceramic, and Plastic Coupons
Small coupons (5 × 5 cm) of stainless steel (grade 304), anti-acid ceramic, and plastic cutting board (high-density polyethylene), which are normally used in the food industry, kitchens, and canteens, were employed to treat the surfaces. They were washed with distilled water and 70% alcohol solution, and then sterilized (14) as well. Next, one of the coupons was sampled with a swab and checked for sterility (15).
Treatment of Coupons by the Best Combination Solutions
According to Salo et al (16), six coupons were placed in a sterile Class III safety cabinet. One milliliter of bacterial suspension was placed at the center of each coupon and spread in the range of one centimeter. All coupons were maintained for 45 minutes to form biofilms.
To evaluate the effect of the disinfectant solution with a sterile hand sprayer containing normal saline as a control or solution (SDS 0.5% + lactic acid 0.02%) at 45°C for S. Typhimurium and (SDS 0.009 + lactic acid + 0.03%) and at 35°C for L. monocytogenes, two puffs (approximately 1 mL) were sprayed on each coupon. After 0, 10, 20, 30, 40, and 60 minutes, the smear site was completely sampled with three sterile swabs, and the samples were transferred to a test tube containing 10 mL of normal saline. The test tube was mixed well, and serial dilutions were prepared for each sample. An aliquot (100 μL) of each dilution was spread on trypticase soy agar, and the plates were incubated at 35°C for 24 hours. The number of colonies grown was counted and calculated based on CFU/mL. All experiments were separately performed for S. Typhimurium and L. monocytogenes on steel, ceramic, and plastic surfaces in triplicate to ensure the accuracy of the results and statistical analysis.
Statistical Analysis
The results were analyzed by one-way and two-way repeated measures ANOVA and LSD post hoc test using SPSS, version 16.0. Differences were considered statistically significant (P ≤ 0.05).
Results
Single Treatments
The results of the repeated measures ANOVA for SDS, citric acid, and lactic acid showed that time, concentration, and interaction time and concentration have a significant effect on the population of S. Typhimurium (P < 0.001). Based on the results, S. Typhimurium had a relative resistance to the bactericidal effects of SDS. As shown in Figure 1A, only 2.5% and 3% of SDS could reduce the population of S. Typhimurium by more than 1 log after 30 minutes. In contrast, 0.2% citric acid produced more than a 1-log reduction in the viability of the bacterium after 10 minutes (Figure 1B), but the same concentration of lactic acid immediately reduced the number of S. Typhimurium by more than 2 logs (Figure 1C). This result indicates that the bactericidal effect of lactic acid is greater than that of citric acid. Figure 1D displays that concentrations of 1.5-4% NaCl did not have a significant effect on reducing the number of S. Typhimurium during the time. Based on the preliminary results, 0.5% SDS, 0.03% citric acid, and 0.02% lactic acid were used for the combined treatments in the next step, and the results were compared at 25°C and 45°C.
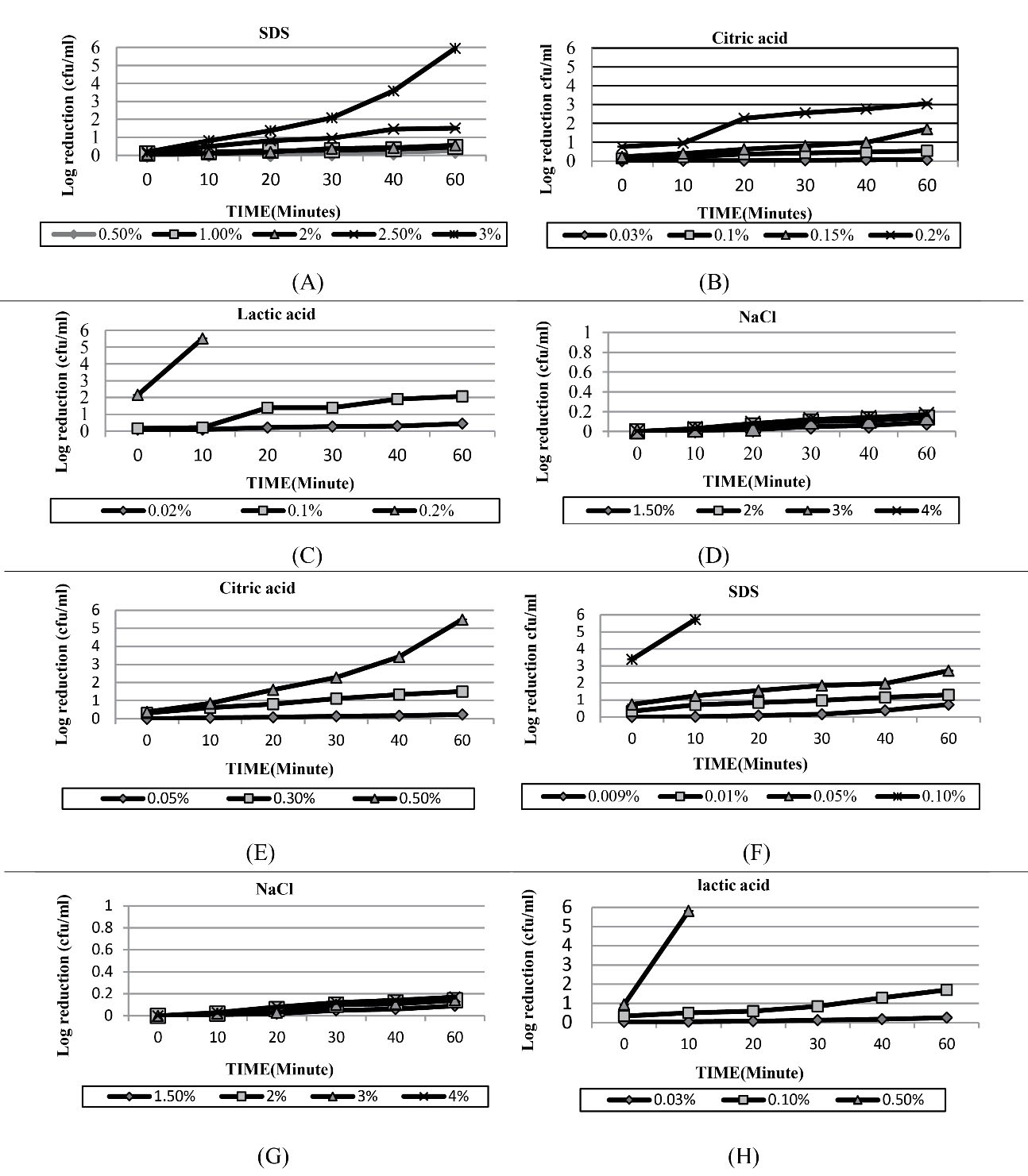
Figure 1.
Bactericidal Effect of Different Concentrations of SDS, Citric Acid, Lactic Acid and NaCl on the Reduction of Bacteria: (A-D) S. Typhimuriumand (E-H) L. monocytogenes. Note. SDS: Sodium dodecyl sulfate
.
Bactericidal Effect of Different Concentrations of SDS, Citric Acid, Lactic Acid and NaCl on the Reduction of Bacteria: (A-D) S. Typhimuriumand (E-H) L. monocytogenes. Note. SDS: Sodium dodecyl sulfate
The statistical analysis of data on SDS, citric acid, and lactic acid demonstrated that time, concentration, and interaction time and concentration exert a significant effect on the population of L. monocytogenes (P < 0.001). Based on the findings, L. monocytogenes was highly sensitive to SDS. According to data in Figure 1E, three logs of the viability of the bacterium were reduced immediately after exposure to SDS, and after 10 minutes, the population of the bacterium reached below the count limit. However, concentrations of 0.01% and 0.009% SDS reduced the number of the bacterium by approximately 1 log after 60 minutes. Concentrations of 0.05% and 0.3% SDS resulted in less than 1- and 2-log reduction in the viability of the bacterium after 60 minutes, respectively.
Based on the preliminary results, 0.5% SDS, 0.03% citric acid, and 0.02% lactic acid were then applied for the combined treatments, and the results were compared at 25°C and 45°C.
The citric acid (0.05%) did not have that much effect on the bacterium load reduction, while the concentrations of 0.3% and 0.5% citric acid produced 1- and 2-log reduction in the viability of the bacterium after 30 minutes (Figure 1F). Likewise, the obtained data revealed that the bactericidal effect of lactic acid was higher than that of citric acid, where the concentration of 0.5% lactic acid produced about 6-log reductions in the viability of the bacterium after 10 minutes (Figure 1G). In addition, none of the tested concentrations of NaCl had a significant effect on the population of the bacterium (Figure 1H). According to the preliminary results, a concentration of 0.009% SDS, 0.05% citric acid, and 0.03% lactic acid was used for the combined treatments in the next step, and the results were compared at 25°C and 35°C during the time.
Combined Treatments
Figure 2A illustrates the results of the treatment of S. Typhimurium with SDS alone or in combination with lactic acid or citric acid at 25°C and 45°C over time. These results showed that the combination of SDS 0.5% with lactic acid 0.02% at 45°C has the best killing effect on S. Typhimurium. Subsequently, the combination of citric acid 0.03% and SDS 0.5% at the same temperature had the greatest effect. As depicted in Figure 1D, the use of NaCl alone had negligible bactericidal effects. The addition of NaCl to the SDS solution also resulted in a severe reduction in SDS bactericidal activity (data are not provided); therefore, NaCl was not used in the subsequent combined treatments.
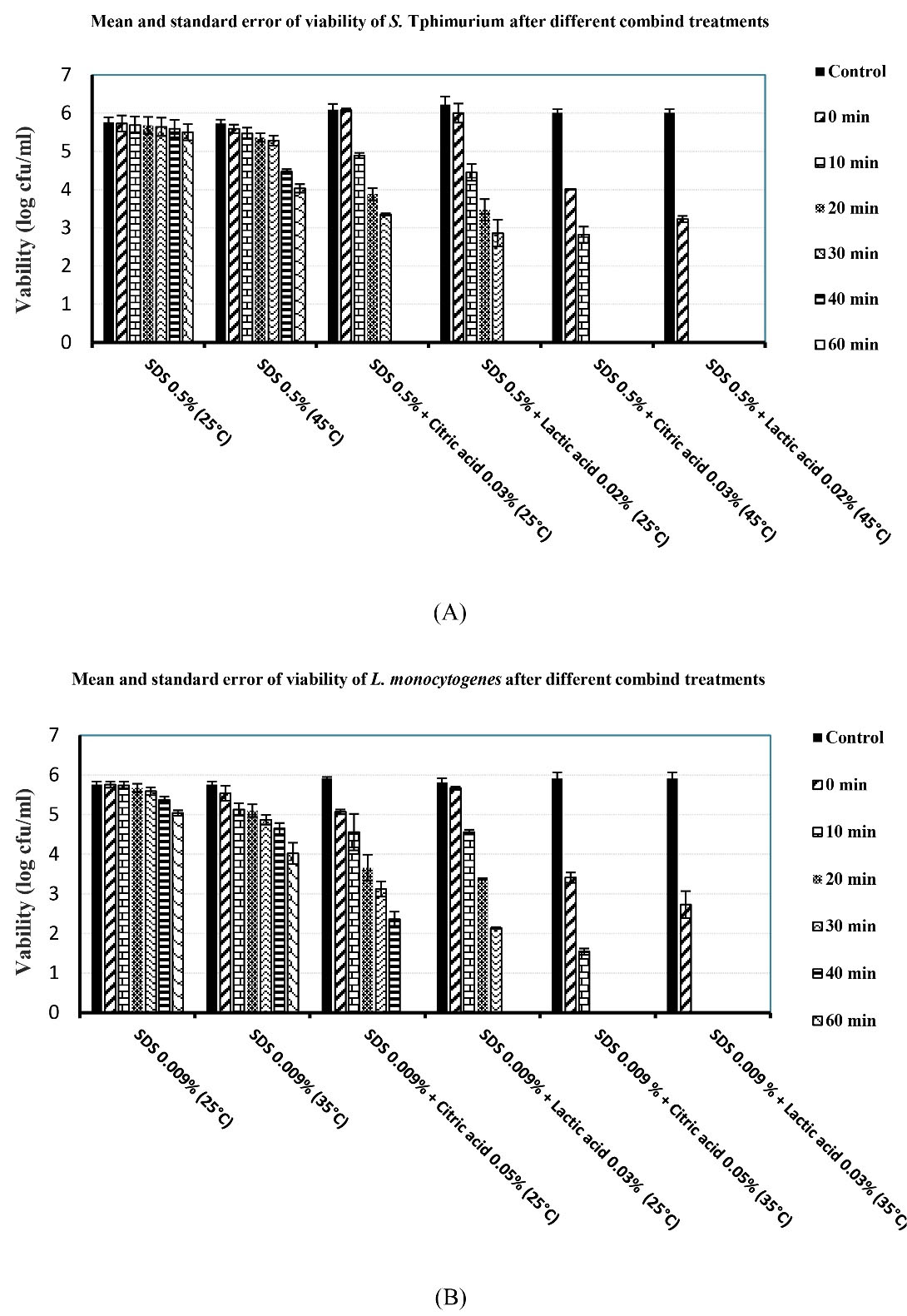
Figure 2.
Bactericidal Effects of Combined Treatments on Bacteria. Note. SDS: Sodium dodecyl sulfate
.
Bactericidal Effects of Combined Treatments on Bacteria. Note. SDS: Sodium dodecyl sulfate
Figure 2B displays the results of the treatment of L. monocytogenes with SDS alone or in combination with lactic acid or citric acid at 25°C and 35°C over time. The results indicated that the combination of a low concentration of SDS (0.009%) with lactic acid 0.03% at 35°C had the best killing effect on L. monocytogenes. Further, the combination of citric acid 0.05% and SDS 0.009% at the same temperature had the greatest effect.
Surface Treatments
Finally, a solution containing SDS 0.5% with lactic acid 0.02% at 45°C was employed to remove S. Typhimurium from the plastic, stainless steel, and ceramic surfaces (Figure 3A). The statistical analysis of data demonstrated that time, surface, and interaction time and surface have a significant effect on the population of S. Typhimurium (P < 0.001). The disinfectant solution completely removed S. Typhimurium from the stainless steel after 10 minutes on the ceramic. Meanwhile, the solution caused approximately 2.5-log reductions in the bacterial population on polyethylene during this time. The bacterium was not detected on the polyethylene surface after 20 minutes.
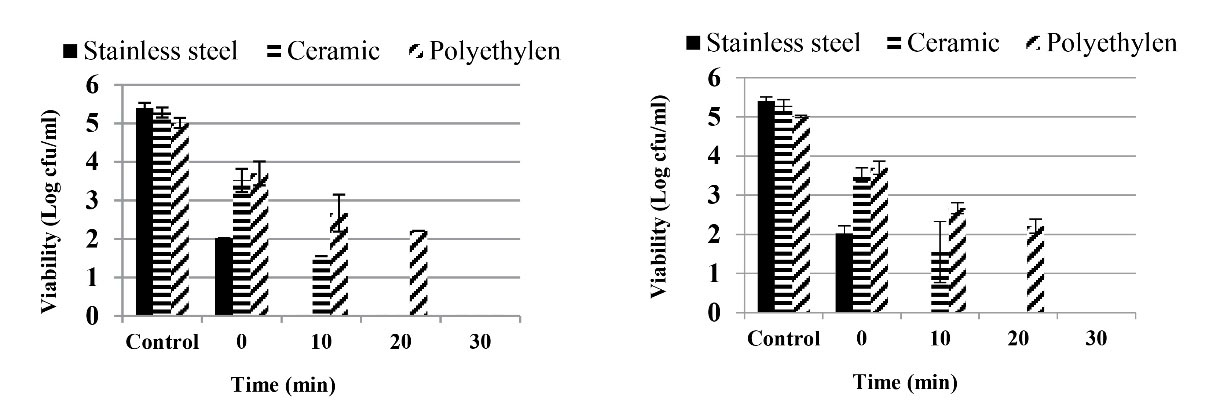
Figure 3.
Viable Population and Standard Error of (A) S. Typhimurium and (B) L. monocytogenes After Treatment by SDS 0.5% With Lactic Acid 0.02% at 45°C for S. Typhimurium and SDS 0.009% + Lactic Acid 0.03% at 35°C for L. monocytogenes on Different Surfaces. Note. SDS: Sodium dodecyl sulfate
.
Viable Population and Standard Error of (A) S. Typhimurium and (B) L. monocytogenes After Treatment by SDS 0.5% With Lactic Acid 0.02% at 45°C for S. Typhimurium and SDS 0.009% + Lactic Acid 0.03% at 35°C for L. monocytogenes on Different Surfaces. Note. SDS: Sodium dodecyl sulfate
To remove L. monocytogenes from plastic, stainless steel, and ceramic surfaces, the best solution (SDS 0.009% + lactic acid 0.03% at 35°C) was used in the final step, and the results are illustrated in Figure 3B. Based on the statistical analysis, time, surface, and interaction time and surface had a significant effect on the population of L. monocytogenes (P < 0.001). The disinfectant solution could completely remove L. monocytogenes from stainless steel after 10 minutes. However, the solution made approximately 2.5- and 3.5-log reductions in the bacterial population on the polyethylene and ceramic after 10 minutes, respectively. The bacterium was not detected on any of the three surfaces after 30 minutes.
Discussion
SDS has been considered a disinfectant for the food industry. The combination of SDS and various substances such as organic acids for food disinfection has received increasing attention in recent years. The antimicrobial efficacy of the combination of SDS and organic acids remains high even when extensively used in food processing environments. The other advantages of using SDS in the food industry are its other properties (e.g., its easy solubility and foaming capacity) and the extension of its potential for surface disinfection and decontamination of food-borne pathogens, including when they form biofilms (17).
Therefore, the composition of SDS and other disinfectants has been studied to increase its bactericidal effect. For example, Lu and Wu (11) found that a combination of thymol, acetic acid, and SDS was more effective than the chlorine solution in reducing Salmonella Enterica in chicken fillets while having little effect on pH and organoleptic properties. Li and Wu (12) successfully used a combination of SDS, acetic acid, and hydrogen peroxide to disinfect raspberries. They reported that a combination of 0.5 mg/mL acetic acid and SDS solution (5000 ppm) or a combination of 200 ppm hydrogen peroxide and 5000 ppm SDS was a suitable alternative to chlorine solution for the disinfection and removal of pathogens such as S. Typhimurium from raspberries.
Instead, some researchers demonstrated that a combination of SDS and substances such as the citric acid has a slight bactericidal effect. In one study, cherry tomatoes were washed with SDS and various additives for 30 minutes. The combined treatment with SDS and LVA represented a synergistic bactericidal effect on the aerobic microflora, total coliform bacteria, and E. coli O157:H7. In contrast, the combination of SDS and other disinfectants such as the citric acid, allyl isothiocyanate, or phytic acid did not reveal a synergistic effect on the bacterial population (18). In another study, the combination of SDS and citric acid or hydrogen peroxide, compared to SDS alone, did not generally result in a significant reduction in the viability of four foodborne pathogens, including L. monocytogenes, S. Typhimurium, S. aureus, and E. coli O157:H7 in eggshells (13).
It should be noted that bacteria behave differently when exposed to SDS, and L. monocytogenes is more sensitive to SDS than E. coli in brine solutions in the food industry (19). The efficacy of LVA in combination with SDS to inactivate S. Typhimurium, L. monocytogenes, and Shiga toxin-producing E. coli in stainless steel biofilms was evaluated as well. The combined activity of LVA and SDS was bactericidal in biofilms against the cells of these three pathogens, and the highest concentrations (3% LVA + 2% SDS) caused the greatest log reduction (20).
This study aimed to minimize the likely negative effects and to observe and compare the synergistic effects of the minimum concentrations of SDS, citric acid, and lactic acid at different temperatures and times. The results showed that the studied bacteria were differently sensitive to different disinfectants, and L. monocytogenes was highly sensitive to SDS, while S. Typhimurium was relatively resistant. Both bacteria were more sensitive to lactic acid than to citric acid, and the bactericidal effects of the combined disinfectant treatments were enhanced at 45°C compared to treatments at 35°C.
Based on the report of a previous study, the presence of NaCl led to a significant decrease in zeta potential, suggesting a reduction in the surface charge of SDS micelles (21). Therefore, a mixture of surfactants and NaCl can be used to reduce the critical micelle concentration (CMC). In this study, the combination of SDS and NaCl reduced the bactericidal effect of SDS. This observation is likely related to the effect of NaCl on SDS, followed by electrostatic repulsion forces and CMC to reduce the effect of SDS. Interestingly, as the salt concentration increased, the bactericidal effect of SDS decreased more strongly. Thus, it is better to use deionized or distilled water for the preparation of disinfectants containing SDS. Water-containing ions or salts can reduce the bactericidal effect of SDS in the preparation of disinfectants.
The bactericidal effects of the selected disinfectant solutions on different surfaces also underwent investigation. The concentrations of the components of the selected disinfectant solution varied based on the sensitivity of each tested bacterium. S. Typhimurium was removed immediately after the disinfectant solution was sprayed on the stainless steel surface. However, due to the extremely low SDS content of the solution used for L. monocytogenes, several viable cells were present on the stainless steel surface. If a higher concentration of SDS is used than for S. Typhimurium and because Listeria is highly sensitive to SDS, the bacterium will also be removed from the stainless steel surface immediately after spraying the disinfectant solution.
To completely remove these two bacteria from ceramic and plastic surfaces, we had to wait more than 20 minutes after spraying the disinfectant solution. This problem may be related to the presence of pores on the uneven surfaces of plastic and ceramics. It has also been shown that bacterial biofilm formation can vary on different surfaces. A previous study investigated the biofilm formation of S. enteritidis on different surfaces in the food industry. The results revealed that bacterial hydrophobicity and surface are important criteria for biofilm formation. The rate of bacterial biofilm formation for 2 hours on glass and steel surfaces was significantly higher (P < 0.05) than on polyethylene surfaces (22).
In this study, most biofilms were formed on stainless steel surfaces. In addition, the rate of biofilm formation on ceramic surfaces was similar to that on polyethylene surfaces, but not significantly different on stainless steel surfaces (P < 0.05). Although biofilm formation was higher on stainless steel surfaces, it appears that immediately after spraying a combined solution containing 0.5% SDS and 0.02% lactic acid at 45°C, bacteria such as Salmonella and Listeria can be removed from stainless steel surfaces. However, to remove these bacteria from surfaces such as ceramics and plastics, it is necessary to wait at least 20 minutes after spraying the disinfectant solution or to use higher concentrations.
Conclusion
The preparation of combined solutions with SDS and organic acid at the appropriate concentration and temperature can be useful in removing or reducing bacterial biofilms on surfaces used in the food industry. The combination of low concentrations of SDS and the lactic acid at 45°C can effectively remove some pathogenic bacteria from a variety of surfaces, especially smooth surfaces such as stainless steel, in a short time. The use of extremely low concentrations of acid solutions in combination with SDS reduces the potential negative effects of these substances and has the best effect on controlling spoilage or pathogenic bacteria in the environment and on food contact surfaces. Therefore, the preparation of such compounds is recommended for surface disinfection. A combined solution containing at least 0.5% SDS, and 0.02% lactic acid at 45°C is recommended as a new disinfectant for the control of asepsis on surfaces in different locations according to the results of this study.
Acknowledgments
We would like to express our appreciation to Mrs. Parisa E. Esfahani for laboratory support and help.
Authors’ Contribution
Conceptualization: Siavash Maktabi.
Data curation: Mehdi Pourmahdi Brojeni.
Formal Analysis: Mehdi Pourmahdi Brojeni.
Investigation: Leila Elahinia.
Project administration: Siavash Maktabi.
Supervision: Siavash Maktabi.
Writing – original draft: Leila Elahinia.
Writing – review & editing: Siavash Maktabi.
Competing Interests
The authors declare that they have no conflict of interests.
Ethical Approval
The experiments were performed according to a protocol approved by the Faculty of Veterinary Medicine, Shahid Chamran University of Ahvaz, Iran.
Funding
This research was financially supported by the Vice Chancellor for Research of Shahid Chamran University of Ahvaz (Grant no: SCU.VF98-534).
References
- Gonzalez-Gonzalez CR, Hindle BJ, Saad S, Stratakos AC. Inactivation of Listeria monocytogenes and Salmonella on stainless steel by a piezoelectric cold atmospheric plasma generator. Appl Sci 2021; 11(8):3567. doi: 10.3390/app11083567 [Crossref] [ Google Scholar]
- Corcoran M, Morris D, De Lappe N, O’Connor J, Lalor P, Dockery P. Commonly used disinfectants fail to eradicate Salmonella enterica biofilms from food contact surface materials. Appl Environ Microbiol 2014; 80(4):1507-14. doi: 10.1128/aem.03109-13 [Crossref] [ Google Scholar]
- Woo IS, Rhee IK, Park HD. Differential damage in bacterial cells by microwave radiation on the basis of cell wall structure. Appl Environ Microbiol 2000; 66(5):2243-7. doi: 10.1128/aem.66.5.2243-2247.2000 [Crossref] [ Google Scholar]
- Zhou M, Doyle MP, Chen D. Combination of levulinic acid and sodium dodecyl sulfate on inactivation of foodborne microorganisms: a review. Crit Rev Food Sci Nutr 2020; 60(15):2526-31. doi: 10.1080/10408398.2019.1650249 [Crossref] [ Google Scholar]
- Zhou Z, Zuber S, Cantergiani F, Butot S, Li D, Stroheker T. Inactivation of viruses and bacteria on strawberries using a levulinic acid plus sodium dodecyl sulfate based sanitizer, taking sensorial and chemical food safety aspects into account. Int J Food Microbiol 2017; 257:176-82. doi: 10.1016/j.ijfoodmicro.2017.06.023 [Crossref] [ Google Scholar]
- Queruau Lamerie T, Nussbaumer S, Décaudin B, Fleury-Souverain S, Goossens JF, Bonnabry P. Evaluation of decontamination efficacy of cleaning solutions on stainless steel and glass surfaces contaminated by 10 antineoplastic agents. Ann Occup Hyg 2013; 57(4):456-69. doi: 10.1093/annhyg/mes087 [Crossref] [ Google Scholar]
- Byelashov OA, Kendall PA, Belk KE, Scanga JA, Sofos JN. Control of Listeria monocytogenes on vacuum-packaged frankfurters sprayed with lactic acid alone or in combination with sodium lauryl sulfate. J Food Prot 2008; 71(4):728-34. doi: 10.4315/0362-028x-71.4.728 [Crossref] [ Google Scholar]
- Zhao T, Zhao P, Doyle MP. Inactivation of Salmonella and Escherichia coli O157:H7 on lettuce and poultry skin by combinations of levulinic acid and sodium dodecyl sulfate. J Food Prot 2009; 72(5):928-36. doi: 10.4315/0362-028x-72.5.928 [Crossref] [ Google Scholar]
- Zhao T, Zhao P, Cannon JL, Doyle MP. Inactivation of Salmonella in biofilms and on chicken cages and preharvest poultry by levulinic acid and sodium dodecyl sulfate. J Food Prot 2011; 74(12):2024-30. doi: 10.4315/0362-028x.jfp-11-197 [Crossref] [ Google Scholar]
- Stelzleni AM, Ponrajan A, Harrison MA. Effects of buffered vinegar and sodium dodecyl sulfate plus levulinic acid on Salmonella Typhimurium survival, shelf-life, and sensory characteristics of ground beef patties. Meat Sci 2013; 95(1):1-7. doi: 10.1016/j.meatsci.2013.04.023 [Crossref] [ Google Scholar]
- Lu Y, Wu C. Reductions of Salmonella enterica on chicken breast by thymol, acetic acid, sodium dodecyl sulfate or hydrogen peroxide combinations as compared to chlorine wash. Int J Food Microbiol 2012; 152(1-2):31-4. doi: 10.1016/j.ijfoodmicro.2011.09.015 [Crossref] [ Google Scholar]
- Li Y, Wu C. Enhanced inactivation of Salmonella Typhimurium from blueberries by combinations of sodium dodecyl sulfate with organic acids or hydrogen peroxide. Food Res Int 2013; 54(2):1553-9. doi: 10.1016/j.foodres.2013.09.012 [Crossref] [ Google Scholar]
- Maktabi S, Zarei M, Rashnavady R. Effect of sequential treatments with sodium dodecyl sulfate and citric acid or hydrogen peroxide on the reduction of some foodborne pathogens on eggshell. Iran J Vet Res 2018; 19(2):113-7. [ Google Scholar]
- Phuvasate S, Su Y-C. Effects of electrolyzed oxidizing water and ice treatments on reducing histamine-producing bacteria on fish skin and food contact surface. Food Control 2010; 21(3):286-91. doi: 10.1016/j.foodcont.2009.06.007 [Crossref] [ Google Scholar]
- Deza MA, Araujo M, Garrido MJ. Inactivation of Escherichia coli, Listeria monocytogenes, Pseudomonas aeruginosa and Staphylococcus aureus on stainless steel and glass surfaces by neutral electrolysed water. Lett Appl Microbiol 2005; 40(5):341-6. doi: 10.1111/j.1472-765X.2005.01679.x [Crossref] [ Google Scholar]
- Salo S, Laine A, Alanko T, Sjöberg AM, Wirtanen G. Validation of the microbiological methods hygicult dipslide, contact plate, and swabbing in surface hygiene control: a Nordic collaborative study. J AOAC Int 2000; 83(6):1357-65. [ Google Scholar]
- Zhou M, Doyle MP, Chen D. Combination of levulinic acid and sodium dodecyl sulfate on inactivation of foodborne microorganisms: a review. Crit Rev Food Sci Nutr 2020; 60(15):2526-31. doi: 10.1080/10408398.2019.1650249 [Crossref] [ Google Scholar]
- Nei D, Okunishi T. Bactericidal effect of sodium dodecyl sulfate in combination with various additives. J NARO Res Dev 2022; 2022(10):47-53. doi: 10.34503/naroj.2022.10_47 [Crossref] [ Google Scholar]
- Maktabi S, Parton R, Watson I. Killing Effect of SDS on Listeria monocytogenes and E. coli in Saline Suspensions Related in Food Industries. Aberdeen, Scotland: Proceeding of Food Microbiology; 2008. p. 221.
- Chen D, Zhao T, Doyle MP. Control of pathogens in biofilms on the surface of stainless steel by levulinic acid plus sodium dodecyl sulfate. Int J Food Microbiol 2015; 207:1-7. doi: 10.1016/j.ijfoodmicro.2015.04.026 [Crossref] [ Google Scholar]
- Xu Q, Nakajima M, Ichikawa S, Nakamura N, Roy P, Okadome H. Effects of surfactant and electrolyte concentrations on bubble formation and stabilization. J Colloid Interface Sci 2009; 332(1):208-14. doi: 10.1016/j.jcis.2008.12.044 [Crossref] [ Google Scholar]
- Mahdavi M, Jalali M, Kermanshahi Roha K. Biofilm formation by Salmonella enteritidis on food contact surfaces. J Biol Sci 2008; 8(2):502-5. doi: 10.3923/jbs.2008.502.505 [Crossref] [ Google Scholar]