Avicenna Journal of Clinical Microbiology and Infection. 9(3):115-118.
doi: 10.34172/ajcmi.2022.3399
Original Article
Royal Jelly Feeding as a Main Inducer of Bcl-2-associated X Protein in the Peripheral Blood Immune Cells of Patients With Chronic Hepatitis B
Morteza Bahaaldin-Beygi 1  , Ashraf Kariminik 1, *
, Ashraf Kariminik 1, *  , Mohammad Kazemi Arababadi 2, 3
, Mohammad Kazemi Arababadi 2, 3 
Author information:
1Department of Microbiology, Kerman Branch, Islamic Azad University, Kerman, Iran
2Immunology of Infectious Diseases Research Center, Research Institute of Basic Medical Sciences, Rafsanjan University of Medical Sciences, Rafsanjan, Iran
3Department of Laboratory Sciences, Faculty of Paramedicine, Rafsanjan University of Medical Sciences, Rafsanjan, Iran
Abstract
Aim: Chemokines, cytokines, and their related molecules play crucial roles in the fight against the hepatitis B virus (HBV) and its related complications. Royal jelly (RJ) is considered an immunomodulatory factor for humans. This clinical trial study aimed to explore the RJ effects on the relative expression of CCL2, CCL3, CCL8, IFN-β, NANOG, OCT4, BAX, and MAVS in chronic hepatitis B (CHB) patients.
Methods: The CHB patients were under one month of RJ treatment, 1 g/d. The relative expressions of CCL2, CCL3, CCL8, IFN-β, NANOG, OCT4, BAX, and MAVS were evaluated using the real-time polymerase chain reaction (PCR) technique.
Results: RJ feeding significantly increased the expression of BAX in the peripheral blood immune cells of CHB patients. However, relative expressions of CCL2, CCL3, CCL8, IFN-β, NANOG, OCT4, and MAVS were not altered following RJ feeding.
Conclusion: RJ can modulate immune responses via induction of homeostasis in the peripheral blood immune cells of CHB patients. Reduced inflammation following RJ feeding may be a result of homeostasis in the peripheral blood immune cells.
Keywords: Chronic hepatitis B, Royal jelly, Chemokine, Gene expression
Copyright and License Information
© 2022 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Bahaaldin-Beygi M, Kariminik A, Kazemi Arababadi M. Royal jelly feeding as a main inducer of bcl2-associated x protein in the peripheral blood immune cells of patients with chronic hepatitis b. Avicenna J Clin Microbiol Infect. 2022; 9(3):115-118. doi:10.34172/ajcmi.2022.3399
Introduction
Cytokines and chemokines are the main parts of the immune responses against the hepatitis B virus (HBV) (1). The molecules play several roles in the immune system, including chemotaxis, angiogenesis/stasis, activation of immune cells, and tissue remodeling (2). Among the chemokines, CC ligand (CCL) 2, 3, and 8, which are known as monocyte chemoattractant protein 1, macrophage inflammatory protein 1-alpha, and monocyte chemoattractant protein 2, respectively, play key roles in the chemotaxis and activation of macrophages against HBV (3,4). Interferon-beta (IFN-β) is a member of interferons, a cytokine family that plays a key anti-viral role against HBV (5). The molecules also participate in HBV-related complications such as liver fibrosis and hepatocellular carcinoma (6,7). Additionally, intracellular viral sensors play key roles in the induction of chemokine and cytokine production. For example, mitochondrial antiviral-signaling protein (MAVS) is a target of a group of cytosolic proteins that detect the presence of the virus (8). Upon binding the cytosolic proteins to MAVS, the sensor will be activated, inducing the virally infected cell to secrete chemokines (8). Therefore, the environmental factors that affect the expression of the molecules during HBV infection can impact the disease compilations. In addition, it has been demonstrated that the Bcl-2-associated X (BAX) protein, NANOG, and octamer binding transcription factor 4 (OCT4) molecules participate in the survival of immune cells and prolonged responses to HBV (9,10). Accordingly, BAX can induce apoptosis, and both NANOG and OCT4 can induce the survival of the cells. Hence, increasing the BAX and decreasing the NANOG and OCT4 expressions are associated with homeostasis and decreased inflammatory responses during chronic hepatitis B (CHB). Therefore, the supplementary foods that alter the expression of the molecules can be considered plausible therapeutic strategies during CHB infection.
It has been reported that a natural product from worker bees (Apis mellifera), royal jelly (RJ) (11), can be used as a dietary supplement (12). RJ plays potentially immunomodulatory roles in the immune system and also has several antimicrobial and antioxidant effects (12-16). Thus, it has been hypothesized that RJ may be useful to regulate immune responses and regulate the molecules involved in HBV-related liver complications. Accordingly, this study was designed to examine the effects of one month of RJ feeding on the relative expressions of CCL2, CCL3, CCL8, IFN-β, NANOG, OCT4, BAX, and MAVS in the peripheral blood immune cells of CHB patients.
Materials and Methods
In this clinical trial study, relative expression of CCL2, CCL3, CCL8, IFN-β, NANOG, OCT4, BAX, and MAVS were explored in 30 (13 men and 17 women) CHB patients who were referred to Samenal-Hojaj hospital in Kerman, Iran. They were under RJ feeding of 0.013 g/kg per day on an average of 1 g/d (17). The patients had normal ranges of liver enzymes (using the commercial kits from MAN Company); hence, they were not under anti-HBV therapy. The patients were entered into the study as the “Guide of Prevention and Treatment in Viral Hepatitis” (18). The patients with microbial co-infections, under treatment of antiviral and immunosuppressive drugs, history of liver disorders, mental disorders, and breastfeeding or pregnancy were excluded from the study. The patient’s blood samples were collected, and the relative expressions of the molecules were evaluated just before and 1 month after RJ feeding (Pars Asal Company, Shiraz, Iran).
RNA Extraction and Complementary DNA Synthesizes
Total mRNA was extracted and converted to complementary DNA using commercial kits from Karmania Pars Gene Company, Kerman, Iran. Briefly, the peripheral blood immune cells were lysed, and then the RNA was precipitated and moved to the high absorbance column. After centrifugation, a wash buffer was used to wash the columns. Finally, the elution buffer was used to collect the total mRNA. The extracted mRNA (1 µg) was added to the 15 µL master mix and adjusted to 20 µL by RNase/DNase-free water, then it was incubated at 40ºC for 60 minutes followed by 5 minutes at 70ºC.
Real-Time Polymerase Chain Reaction
Relative expression of CCL2, CCL3, CCL8, IFN-β, NANOG, OCT4, BAX, and MAVS was carried out using SYBR Green real-time polymerase chain reaction (PCR) technique. Consequently, a master mix was used in a Rotor-Gene Q instrument using the following program: 95ºC for 3 minutes and then 40 cycles with 95ºC for 15 seconds/60ºC for 35 seconds, followed by a melting curve ranging from 60ºC to 95ºC (acquiring fluorescence data every 0.3ºC). This master mix was obtained from Biosystem Company in association with specific primers (Table 1) and was designed by Primer 3 software. To verify specific amplification, in addition to the melting curve step during the run, the assay also confirmed the amplicon sizes by 1% agarose gel electrophoresis. Finally, the raw data were analyzed by the 2-∆∆Ct formula using glyceraldehyde-3-phosphate dehydrogenase as a housekeeping gene.
Table 1.
Primer Sequences Used in Real-time PCR Assays
|
Gene
|
Primer Sequences (5'-3')
|
| CCL2 |
ATGAAAGTCTCTGCCGCCCTTCTGT
AGTCTTCGGAGTTTGGGTTTGCTTG |
| CCL3 |
ATGCAGGTCTCCACTGCTGCCCTT
GCACTCAGCTCCAGGTCGCTGACAT |
| CCL8 |
TATCCAGAGGCTGGAGAGCTAC
TGGAATCCCTGACCCATCTCTC |
| IFN-β |
CTTGGATTCCTACAAAGAAGCAGC
TCCTCCTTCTGGAACTGCTGCA |
| NANOG |
ATACCTCAGCCTCCAGCAGA
GCTCCAGGTTGAATTGTTCC |
| OCT4 |
ATTCAGCCAAACGACCATCT
TCTCCAGGTTGCCTCTCACT |
| BAX |
CCAAGAAGCTGAGCGAGTGT
CAGTTGAAGTTGCCGTCAGA |
| MAVS |
AGCAAGAGACCAGGATCGAC
GGGTATTGAAGAGATGCCAGAG |
| GAPDH |
GGATTTGGTCGTATTGGG
GGAAGATGGTGATGGGATT |
Note. PCR: Polymerase chain reaction; CCL: CC Ligand;IFN-β: Interferon-beta; BAX: Bcl-2-associated X; OCT4: Octamer binding transcription factor; MAVS: Mitochondrial antiviral signaling.
Data Analysis and Statistical Methods
Data analysis was performed using SPSS software version 18 and the dependent paired t test to analyze mRNA levels of CCL2, CCL3, CCL8, IFN-β, NANOG, OCT4, BAX, and MAVS before and after RJ feeding. P value was considered significant at < 0.05.
Results
The results indicated that feeding with RJ led to significant upregulation of BAX (P = 0.003). Accordingly, relative expressions of BAX before and after RJ feeding were 1.00 ± 0.43 and 3.84 ± 0.84, respectively. However, RJ feeding did not alter relative expression of CCL2 (P = 0.160), CCL3 (P = 0.997), CCL8 (P = 0.237), IFN-β (P = 0.083), NANOG (P = 0.660), OCT4 (P = 0.519), and MAVS (P = 0.155) in a significant manner. Figure 1 presents the relative expression of the molecules before and after RJ feeding in detail.
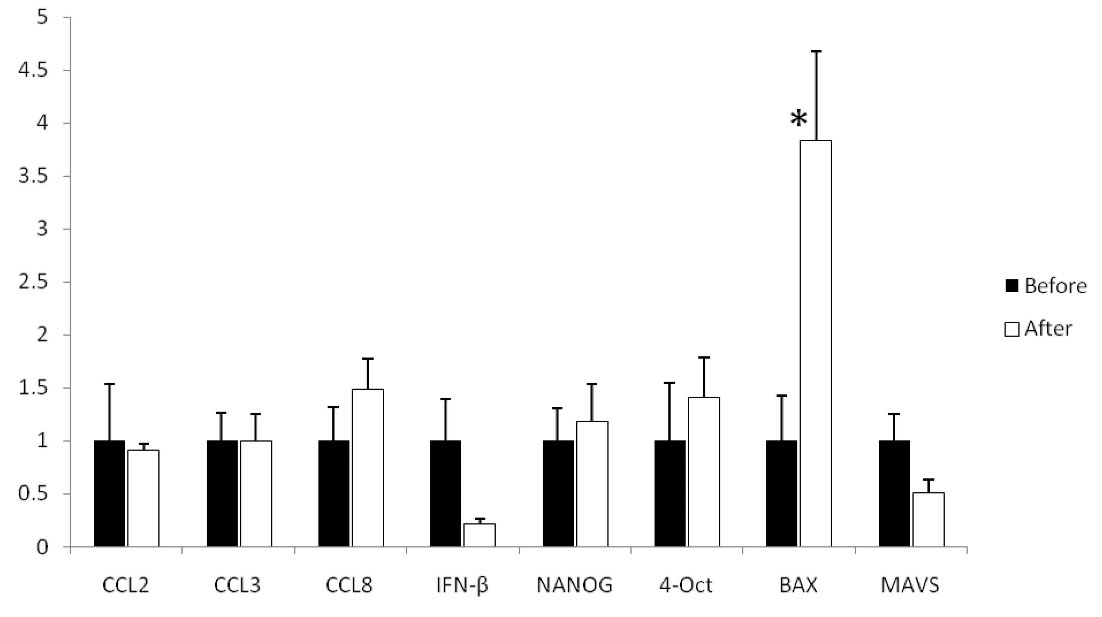
Figure 1.
Relative expression of CCL2, CCL3, CCL8, IFN-β, NANOG, OCT4, BAX, and MAVS Before and after RJ Feeding. The paired t test revealed that the relative expression of BAX significantly increased after one month of RJ feeding. Relative expression of CCL2, CCL3, CCL8, IFN-β, NANOG, OCT4, and MAVS was not different when compared to before and after RJ feeding. Note. CCL: CC Ligand;IFN-β: Interferon-beta; BAX: Bcl-2-associated X; OCT4: Octamer binding transcription factor; MAVS: Mitochondrial antiviral signaling
.
Relative expression of CCL2, CCL3, CCL8, IFN-β, NANOG, OCT4, BAX, and MAVS Before and after RJ Feeding. The paired t test revealed that the relative expression of BAX significantly increased after one month of RJ feeding. Relative expression of CCL2, CCL3, CCL8, IFN-β, NANOG, OCT4, and MAVS was not different when compared to before and after RJ feeding. Note. CCL: CC Ligand;IFN-β: Interferon-beta; BAX: Bcl-2-associated X; OCT4: Octamer binding transcription factor; MAVS: Mitochondrial antiviral signaling
Discussion
The results demonstrated that RJ feeding led to significantly increased expression of BAX in the peripheral blood immune cells. However, RJ feeding was not associated with significant alteration in the expression of the chemokines, IFN-β, NANOG, OCT4, and MAVS. Since BAX plays a key role in the induction of apoptosis, upregulation of the molecule following RJ feeding demonstrated that RJ can induce homeostasis in the activated immune cells and modulate immune responses independent of CCL2, CCL3, CCL8, IFN-β, NANOG, OCT4, and MAVS. To the best of our knowledge, this is the first study on RJ feeding in CHB patients to explore the expression of CCL2, CCL3, CCL8, IFN-β, NANOG, OCT4, BAX, and MAVS molecules. However, the anti-HBV effects of RJ have been reported previously (19,20). RJ also plays significant roles in reducing inflammation, protecting the liver, and improving its functions during CHB and non-infectious diseases (21-23). It appears that RJ applies several mechanisms to reduce inflammation. However, according to the results of the present study, it appears that RJ increases apoptosis in the human peripheral blood immune cells to induce homeostasis and decreases the number of immune cells to reduce inflammation. In line with the current results, an in vitro investigation revealed that RJ increases BAX levels in human lymphocytes (24). Another animal model also confirmed the roles played by RJ in increasing the expression of BAX in the heart tissue (25). However, several reports demonstrated that RJ can decrease the expression of BAX in a dose-dependent manner (26,27). According to the best of our knowledge, the present study is unique as it was performed on CHB patients; thus, it may be concluded that the responses of the immune cells for expression of BAX with regard to the effects of RJ are dependent on the infection with HBV. Accordingly, in physiological conditions, RJ may lead to decreased apoptosis as reported by Veshkini et al (26) and Hashem et al (27). Therefore, it may be concluded that the effects of RJ on the expression of BAX are dose-dependent and also depend on the pathological/physiological conditions. According to the obtained results in this study, it may be concluded that RJ is an inducer of apoptosis in the human blood immune cells in 1 gram/day dose, which may decrease inflammation in CHB patients. Previous investigations proved that RJ uses several mechanisms, including suppression of nuclear factor kappa-light-chain-enhancer of activated B cells and other related signaling pathway phosphorylation (28-31).
Conclusion
It seems that RJ effects on the immune system of HBV-infected patients are complicated and need more investigations. Based on the obtained results, RJ is unable to alter the expression of the IFN-β, CCL2, CCL3, and CCL8 as parts of immune responses against HBV. Additionally, the cytokine and chemokines are involved in inflammation; hence, it appears that the immunoregulatory effects of RJ are independent of the molecules. Moreover, the survival of immune cells during RJ feeding is not affected by alteration in the expression of OCT4 and NANOG. The recognition of HBV molecules by MAVS is not also impacted by RJ feeding. Due to the sample size of the present study, the increased sample size and evaluation of the molecules at the protein levels may be associated with diverse factors.
Acknowledgments
The authors are grateful to the staff of Samenal-Hojaj hospital of Kerman, Iran, who helped collect samples for this study.
Conflict of Interests
The authors declare that they have no known competing financial interests or personal relationships that could apparently influence the work reported in this study.
Ethical Approval
The Ethical Committee of Kerman University of Medical Sciences (IR.IAU.Kerman.REC.1400.010) and the Iranian Registry of Clinical Trials approved the project protocol (IRCT20210620051640N1).
References
- Khorramdelazad H, Hassanshahi G, Kazemi Arababadi M. Controversial issues regarding the roles of IL-10 and IFN-γ in active/inactive chronic hepatitis B. World J Gastrointest Pathophysiol 2014; 5(2):120-1. doi: 10.4291/wjgp.v5.i2.120 [Crossref] [ Google Scholar]
- Hassanshahi G, Kazemi Arababadi M, Khoramdelazad H, Yaghini N, Rezazadeh Zarandi E. Assessment of CXCL12 (SDF-1α) polymorphisms and its serum level in posttransfusion occult HBV-infected patients in Southeastern Iran. Arch Med Res 2010; 41(5):338-42. doi: 10.1016/j.arcmed.2010.07.001 [Crossref] [ Google Scholar]
- Tan AT, Koh S, Goh W, Zhe HY, Gehring AJ, Lim SG. A longitudinal analysis of innate and adaptive immune profile during hepatic flares in chronic hepatitis B. J Hepatol 2010; 52(3):330-9. doi: 10.1016/j.jhep.2009.12.015 [Crossref] [ Google Scholar]
- Yoshio S, Mano Y, Doi H, Shoji H, Shimagaki T, Sakamoto Y. Cytokine and chemokine signatures associated with hepatitis B surface antigen loss in hepatitis B patients. JCI Insight 2018; 3(20):e122268. doi: 10.1172/jci.insight.122268 [Crossref] [ Google Scholar]
- Kayesh MEH, Ezzikouri S, Chi H, Sanada T, Yamamoto N, Kitab B. Interferon-β response is impaired by hepatitis B virus infection in Tupaia belangeri. Virus Res 2017; 237:47-57. doi: 10.1016/j.virusres.2017.05.013 [Crossref] [ Google Scholar]
- Mani SKK, Andrisani O. Interferon signaling during hepatitis B virus (HBV) infection and HBV-associated hepatocellular carcinoma. Cytokine 2019; 124:154518. doi: 10.1016/j.cyto.2018.08.012 [Crossref] [ Google Scholar]
- Zhuang H, Cao G, Kou C, Liu T. CCL2/CCR2 axis induces hepatocellular carcinoma invasion and epithelial-mesenchymal transition in vitro through activation of the Hedgehog pathway. Oncol Rep 2018; 39(1):21-30. doi: 10.3892/or.2017.6069 [Crossref] [ Google Scholar]
- Wei C, Ni C, Song T, Liu Y, Yang X, Zheng Z. The hepatitis B virus X protein disrupts innate immunity by downregulating mitochondrial antiviral signaling protein. J Immunol 2010; 185(2):1158-68. doi: 10.4049/jimmunol.0903874 [Crossref] [ Google Scholar]
- Li W, Duan X, Zhu C, Liu X, Jeyarajan AJ, Xu M. Hepatitis B and hepatitis C virus infection promote liver fibrogenesis through a TGF-β1-induced OCT4/Nanog pathway. J Immunol 2022; 208(3):672-84. doi: 10.4049/jimmunol.2001453 [Crossref] [ Google Scholar]
- Song CZ, Wang QW, Song CC, Bai ZL. Viral replication modulated by synthetic peptide derived from hepatitis B virus X protein. World J Gastroenterol 2004; 10(3):389-92. doi: 10.3748/wjg.v10.i3.389 [Crossref] [ Google Scholar]
- Kamakura M. Royalactin induces queen differentiation in honeybees. Nature 2011; 473(7348):478-83. doi: 10.1038/nature10093 [Crossref] [ Google Scholar]
- Ramadan MF, Al-Ghamdi A. Bioactive compounds and health-promoting properties of royal jelly: a review. J Funct Foods 2012; 4(1):39-52. doi: 10.1016/j.jff.2011.12.007 [Crossref] [ Google Scholar]
- Pavel CI, Mărghitaş LA, Bobiş O, Dezmirean DS, Şapcaliu A, Radoi I. Biological activities of royal jelly-review. Sci Papers Anim Sci Biotechnol 2011; 44(2):108-18. [ Google Scholar]
- El-Gayar MH, Aboshanab KM, Aboulwafa MM, Hassouna NA. Antivirulence and wound healing effects of royal jelly and garlic extract for the control of MRSA skin infections. Wound Med 2016; 13:18-27. doi: 10.1016/j.wndm.2016.05.004 [Crossref] [ Google Scholar]
- Nascimento AP, Moraes LA, Ferreira NU, de Padua Moreno G, Uahib FG, Barizon EA. The lyophilization process maintains the chemical and biological characteristics of royal jelly. Evid Based Complement Alternat Med 2015; 2015:825068. doi: 10.1155/2015/825068 [Crossref] [ Google Scholar]
- Delkhoshe-Kasmaie F, Malekinejad H, Khoramjouy M, Rezaei-Golmisheh A, Janbaze-Acyabar H. Royal jelly protects from taxol-induced testicular damages via improvement of antioxidant status and up-regulation of E2f1. Syst Biol Reprod Med 2014; 60(2):80-8. doi: 10.3109/19396368.2013.852271 [Crossref] [ Google Scholar]
- Pourmobini H, Kazemi Arababadi M, Salahshoor MR, Roshankhah S, Taghavi MM, Taghipour Z. The effect of royal jelly and silver nanoparticles on liver and kidney inflammation. Avicenna J Phytomed 2021; 11(3):218-23. [ Google Scholar]
- Liu HG, Chen WW, Fan ZP, Yang HY, Shi M, Zhang Z. The high prevalence of the I27 mutant HBcAg18-27 epitope in Chinese HBV-infected patients and its cross-reactivity with the V27 prototype epitope. Clin Immunol 2007; 125(3):337-45. doi: 10.1016/j.clim.2007.06.010 [Crossref] [ Google Scholar]
- Habashy NH, Abu-Serie MM. Major royal-jelly protein 2 and its isoform X1 are two novel safe inhibitors for hepatitis C and B viral entry and replication. Int J Biol Macromol 2019; 141:1072-87. doi: 10.1016/j.ijbiomac.2019.09.080 [Crossref] [ Google Scholar]
- Fatima I, Kanwal S, Mahmood T. Natural products mediated targeting of virally infected cancer. Dose Response 2019; 17(1):1559325818813227. doi: 10.1177/1559325818813227 [Crossref] [ Google Scholar]
- Blank S, Bantleon FI, McIntyre M, Ollert M, Spillner E. The major royal jelly proteins 8 and 9 (Api m 11) are glycosylated components of Apis mellifera venom with allergenic potential beyond carbohydrate-based reactivity. Clin Exp Allergy 2012; 42(6):976-85. doi: 10.1111/j.1365-2222.2012.03966.x [Crossref] [ Google Scholar]
- Cornara L, Biagi M, Xiao J, Burlando B. Therapeutic properties of bioactive compounds from different honeybee products. Front Pharmacol 2017; 8:412. doi: 10.3389/fphar.2017.00412 [Crossref] [ Google Scholar]
- Kohno K, Okamoto I, Sano O, Arai N, Iwaki K, Ikeda M. Royal jelly inhibits the production of proinflammatory cytokines by activated macrophages. Biosci Biotechnol Biochem 2004; 68(1):138-45. doi: 10.1271/bbb.68.138 [Crossref] [ Google Scholar]
- Jenkhetkan W, Thitiorul S, Jansom C, Ratanavalachai T. Molecular and cytogenetic effects of Thai royal jelly: modulation through c-MYC, h-TERT, NRF2, HO-1, BCL2, BAX and cyclins in human lymphocytes in vitro. Mutagenesis 2017; 32(5):525-31. doi: 10.1093/mutage/gex020 [Crossref] [ Google Scholar]
- Aslan A, Beyaz S, Gok O, Can MI, Parlak G, Ozercan IH. Royal jelly abrogates flouride-induced oxidative damage in rat heart tissue by activating of the nrf-2/NF-κB and bcl-2/bax pathway. Toxicol Mech Methods 2021; 31(9):644-54. doi: 10.1080/15376516.2021.1950249 [Crossref] [ Google Scholar]
- Veshkini A, Mohammadi-Sangcheshmeh A, Ghanem N, Abazari-Kia AH, Mottaghi E, Kamaledini R. Oocyte maturation with royal jelly increases embryo development and reduces apoptosis in goats. Anim Reprod 2018; 15(2):124-34. doi: 10.21451/1984-3143-2017-ar986 [Crossref] [ Google Scholar]
- Hashem KS, Elkelawy A, Abd-Allah S, Helmy NA. Involvement of Mfn2, Bcl2/Bax signaling and mitochondrial viability in the potential protective effect of royal jelly against mitochondria-mediated ovarian apoptosis by cisplatin in rats. Iran J Basic Med Sci 2020; 23(4):515-26. doi: 10.22038/ijbms.2020.40401.9563 [Crossref] [ Google Scholar]
- Ali AM, Kunugi H. Bee honey protects astrocytes against oxidative stress: a preliminary in vitro investigation. Neuropsychopharmacol Rep 2019; 39(4):312-4. doi: 10.1002/npr2.12079 [Crossref] [ Google Scholar]
- Kawahata I, Xu H, Takahashi M, Murata K, Han W, Yamaguchi Y. Royal jelly coordinately enhances hippocampal neuronal expression of somatostatin and neprilysin genes conferring neuronal protection against toxic soluble amyloid-β oligomers implicated in Alzheimer’s disease pathogenesis. J Funct Foods 2018; 51:28-38. doi: 10.1016/j.jff.2018.10.006 [Crossref] [ Google Scholar]
- Mohamed AA, Galal AAA, Elewa YHA. Comparative protective effects of royal jelly and cod liver oil against neurotoxic impact of tartrazine on male rat pups brain. Acta Histochem 2015; 117(7):649-58. doi: 10.1016/j.acthis.2015.07.002 [Crossref] [ Google Scholar]
- You MM, Chen YF, Pan YM, Liu YC, Tu J, Wang K. Royal jelly attenuates LPS-induced inflammation in BV-2 microglial cells through modulating NF-κB and p38/JNK signaling pathways. Mediators Inflamm 2018; 2018:7834381. doi: 10.1155/2018/7834381 [Crossref] [ Google Scholar]