Avicenna Journal of Clinical Microbiology and Infection. 9(4):137-147.
doi: 10.34172/ajcmi.2022.3391
Original Article
Epidemiology and Antibiogram of Clinical Staphylococcus aureus Isolates from Tertiary Care Hospitals in Dhaka, Bangladesh
Fatema Mohammad Alam 1, *  , Tamanna Tasnim 1
, Tamanna Tasnim 1  , Sonia Afroz 1
, Sonia Afroz 1  , Abdur Rahman Mohammad Alam 2
, Abdur Rahman Mohammad Alam 2  , Nabila Afroze 3
, Nabila Afroze 3  , Aysha Khatun 1, Sanjida Khondakar Setu 1, Ahmed Abu Saleh 1
, Aysha Khatun 1, Sanjida Khondakar Setu 1, Ahmed Abu Saleh 1
Author information:
1Department of Microbiology and Immunology, Bangabandhu Sheikh Mujib Medical University, Dhaka 1000, Bangladesh
2Department of Ophthalmology, Bangabandhu Sheikh Mujib Medical University, Dhaka 1000, Bangladesh
3Department of Vitreo-Retina, National Institute of Ophthalmology and Hospital, Dhaka 1207, Bangladesh
Abstract
Background: This study aimed to investigate the epidemiology and antibiogram of clinical Staphylococcus aureus isolates from three tertiary care hospitals in Dhaka, Bangladesh.
Methods: A total of 185 clinical S. aureus isolates were studied from March 2016 to February 2017 and identified by standard microbiological methods and an antibiogram was determined by disc diffusion method. A duplex polymerase chain reaction (PCR) assay was performed on all isolates to detect femA and mecA genes of S. aureus.
Results: Among the 185 isolates, all (100%) were positive for the femA gene, 76 (41.1%) were methicillinresistant S. aureus (MRSA), and 109 (58.9%) were methicillin-susceptible S. aureus (MSSA). The highest and the lowest frequency of both MRSA were isolated from pus and urine specimens, respectively. All 185 S. aureus were 100% sensitive to both vancomycin and linezolid and were highly sensitive towards rifampicin (94%), meropenem (87%), gentamicin (85.4%), and cotrimoxazole (82.2%), whereas the highest resistance was against penicillin G (94.6%) followed by amoxicillin/clavulanic acid (82.7%), azithromycin (72.4%), amoxicillin (66.5%), and ciprofloxacin (63.2%). After vancomycin and linezolid, MRSA showed good susceptibility to rifampicin, cotrimoxazole, and gentamicin, while MSSA exhibited high sensitivity toward rifampicin, gentamicin, cefoxitin, meropenem, cloxacillin, ceftriaxone, and cotrimoxazole. Furthermore, MRSA was significantly more resistant to antibiotics than MSSA (P value<0.05), and the majority of S. aureus (81.1%), MRSA (97.4%), and MSSA (69.7%) were multidrug-resistant (MDR).
Conclusion: Our findings can guide physicians to provide effective antibiotic therapy, implement monitoring and control strategies to reduce antimicrobial resistance, and prevent the dissemination of MRSA and MDR in the environment.
Keywords:
Staphylococcus aureus, mecA gene, Methicillin-resistant Staphylococcus aureus, Methicillin sensitive Staphylococcus aureus, Antibiogram, Multidrug resistant
Copyright and License Information
© 2022 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Alam FM, Tasnim T, Afroz S, Alam ARM, Afroze N, Khatun A, et al. Epidemiology and antibiogram of clinical Staphylococcus aureus isolates from tertiary care hospitals in Dhaka, Bangladesh. Avicenna J Clin Microbiol Infect. 2022; 9(4):137-147. doi:10.34172/ajcmi.2022.3391
Introduction
Staphylococcus aureus is one of the most successful and adaptable human pathogens and a major cause of infections in both hospital and community settings, ranging from minor skin diseases to life-threatening invasive infections (1,2). It has also been found to have the extraordinary capability to react to each new antibiotic swiftly by developing a resistance mechanism, which is a major clinical hindrance in treating infections (3).
Infections are caused by both methicillin-susceptible S. aureus (MSSA) and methicillin-resistant S. aureus (MRSA) strains. MRSA is by definition resistant to beta-lactams antibiotics regardless of its in vitro susceptibility results, involving all categories of penicillins, cephalosporins, beta-lactamase inhibitors, and carbapenems with the exception of the anti-MRSA cephalosporins (4-6). The first case of MRSA was reported in England, just two years after the introduction of methicillin in 1959, and today it has become prevalent worldwide (7). A systemic review in Bangladesh reported the prevalence of MRSA in all culture isolates to range from 4.8% to 78.7% (8).
The genome of MRSA bears the staphylococcal cassette chromosome meccontaining the mecA gene which is responsible for methicillin resistance. The mecA gene encodes the penicillin-binding protein (PBP) 2a or PBP-2a, which has a low affinity for beta-lactam antibiotics in addition to other resistance determinants (9,10). To identify MRSA, mecA gene detection is regarded as the gold standard method (11-13). The femA gene is specific to and universally present in all S. aureus isolates, encoding a factor that has been postulated to have a role in cell wall metabolism (10,14,15). Therefore, the detection of the femA and mecA in the same organism can lead to the identification of the species and drug resistance phenotype.
The few antibiotics which are currently available to treat MRSA include glycopeptides (e.g., vancomycin and teicoplanin), linezolid (an oxazolidinone), tigecycline, daptomycin, telavancin, Synercid (a combination of quinupristin and dalfopristin), and anti-MRSA cephalosporins (e.g., ceftaroline and ceftobiprole), among which vancomycin followed by linezolid remain as the cornerstone therapeutic options for MRSA infection treatment (1,16).
The degree of resistance, which might not stay constant over time, is a crucial factor to take into account when selecting an antibiotic (17). Studies from Bangladesh and other countries across the world have documented changes in the drug susceptibility profile of S. aureus (18-20). The emergence of multidrug-resistant (MDR) strains of S. aureus, which are known to develop non-susceptibility to at least one agent in three or more antimicrobial categories, has made the treatment of S. aureus infections more challenging (2,5,7). Moreover, the emergence of vancomycin resistance in addition to resistance against a broad array of structurally unrelated antimicrobials has made MRSA become an MDR superbug, increasing its risk in both the hospital and community environment (1,7).
Therefore, knowledge of the epidemiologic trends and antibiotic susceptibility patterns of S. aureus in a local setting becomes fundamental. Based on this background, the present study aimed to investigate the epidemiology and antibiogram of clinical S. aureus isolates collected from three tertiary care hospitals in Dhaka, Bangladesh. The epidemiology of MRSA and MSSA isolates was studied by determining the overall prevalence and prevalence in the different tertiary care hospitals and different clinical specimens. Accordingly, the in vitro susceptibility to antibiotics was tested, and the prevalence of MDR isolates among MRSA and MSSA was determined.
Materials and Methods
Collection of Bacterial Isolates and Study Place
A total of 185 consecutive non-duplicate clinical isolates of S. aureus were studied from March 2016 to February 2017. The suspected S. aureus isolates recovered from different clinical specimens (e.g., pus, wound swab, blood, and urine) were collected from the Microbiology laboratories of three tertiary care hospitals, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka Medical College (DMC), Bangladesh Institute of Research and Rehabilitation in Diabetes, Endocrine, and Metabolic Disorders (BIRDEM). All three hospitals are located in close proximity to each other in Dhaka and provide healthcare services to patients from all over the country. Relevant clinical data for each bacterial specimen was obtained from laboratory records and recorded in a predesigned data sheet.All laboratory procedures were performed in the department of Microbiology and Immunology, BSMMU, Dhaka.
Identification of Staphylococcus aureus Isolates
The isolates were at first inoculated into blood agar media (Oxoid Limited, Basingstoke, Hampshire, United Kingdom) and incubated aerobically at 37°C for 24 hours to ensure purity and viability (21). Then, the bacterial growths were identified as S. aureus by colony morphology, haemolytic property, and pigment production on blood agar media, gram staining, catalase test, coagulase test (slide and tube method), and mannitol fermentation test in mannitol salt agar media (Oxoid Limited, Basingstoke, Hampshire, United Kingdom) as per standard methods (22,23). The findings were confirmed by the detection of femA gene via polymerase chain reaction (PCR) assay. All the isolates were preserved in nutrient agar slant (Oxoid Limited, Basingstoke, Hampshire, United Kingdom) inside screw-capped vials and stored at 4°C temperature for further molecular study.
Antimicrobial Susceptibility Test and Phenotypic Detection of MRSA Strains by Cefoxitin Disc Diffusion Test
Antimicrobial susceptibility testing of the 185 S. aureus isolates and the phenotypic detection of MRSA by cefoxitin disc diffusion test (CDDT) were carried out by Kirby-Bauer disc diffusion method on the same Mueller-Hinton agar medium (Oxoid Limited, Basingstoke, Hampshire, United Kingdom) as per the Clinical and Laboratory Standards Institute (CLSI, 2014) recommendations (4). The isolates were tested for susceptibility against the antibiotics such aspenicillin G (10µg), cloxacillin (1 µg), amoxicillin (30 µg), amoxicillin/clavulanic acid (20/10 µg), ceftriaxone (30 µg), cefoxitin (30 µg), meropenem (10 µg), gentamicin (10 µg), azithromycin (30 µg), cotrimoxazole (1.25/23.75 µg), ciprofloxacin (5 µg), rifampicin (5 µg), vancomycin (30 µg), and linezolid (30 µg). Antibiotic discs were purchased from HiMedia (HiMedia Laboratories, Mumbai, India). Briefly, theMueller-Hinton agar plates were inoculated with bacterial suspension adjusted withthe turbidity of a 0.5 McFarland standard tube and left on the level surface for 10 to 15 minutes, and discs were then placed on the inoculated surface at an appropriate distance using sterile forceps. The plates were then incubated at 37°C for 24 hours. The following day, the reading of the inhibition zone was taken by measuring scale against good light and noted in millimeters, and thezone ofinhibition was compared with the CLSIrecommended standard values and recorded as sensitive or resistant (4). The findings of CDDT were confirmed by the detection of the mecA gene through PCR assay, and S. aureus ATCC 25923 was utilized as a reference control organism.
Identification of Multi-Drug Resistant Staphylococcus aureus on Phenotypic Basis
Based on the antibiotic susceptibility results, S. aureus isolates that fulfilled either one or both of the following criteria were categorized as MDR (5):
-
Resistance to cefoxitin: Here, cefoxitin represents all β-lactams and predicts non-susceptibility to all categories of β-lactam antimicrobials (i.e., penicillins, cephalosporins/cephamycins, β-lactamase inhibitors, and carbapenems) with the exception of the anti-MRSA cephalosporins.
-
Non-susceptible to one or more agents in three or more antimicrobial categories.
In this study, antibiotics from the following antimicrobial categories were tested: penicillins, cephalosporins/cephamycins, β-lactamase inhibitors, carbapenems, aminoglycosides, macrolides, folate pathway inhibitors, fluoroquinolones, ansamycins, glycopeptides, and oxazolidinones.
Polymerase Chain Reaction
The duplex polymerase chain reaction (PCR) assay was carried out on all of the study isolates for the detection of the S. aureus genes:femAandmecA. The detection of the femA gene was used to confirm the identity of S. aureus, and it was also used as an internal positive control to validate the PCR conditions (14,24). Moreover, the detection of the mecA gene was used as the confirmatory method for the identification of the MRSA strains.As a negative control, sterile water (1 µL) was tested during each PCR run (14).
( i ) DNA Extraction from Bacterial Pellets
At first, preserved colonies from the nutrient agar slants were thawed and inoculated onto blood agar plates and incubated at 37°C for 18 hours to ensure purity. Single colonies from here were taken and inoculated into 0.5 ml brain heart infusion broth (HiMedia Laboratories, Mumbai, India) in a sterile 1.5 mL microcentrifuge tube and incubated overnight at 37°Ctemperature. Next, the total DNA was extracted from the broth cultures by using the QIAamp DNA mini kit (Qiagen, Hilden, Germany) in accordance with the manufacturer’s instructions for gram-positive bacteria. The extracted DNA was kept in microcentrifuge tubes and stored at -20°Cuntil PCR assay.
(ii) Primers Used for the Duplex PCR Assay
The duplex PCR assay was carried out using femAand mecA gene-specific primers previously described byMehrotra et al (14) and synthesized by Integrated DNA Technologies (New York, USA). The primer sequences are presented in Table 1.
Table 1.
The Oligonucleotide Primer Sequences Used for Amplification of the S. aureus Genes
|
Target Gene/ Primer
|
Primer Sequences (5’-3’)
|
Size of Amplified Product (bp)*
|
Reference
|
|
femA
|
AAAAAAGCACATAACAAGCG
GATAAAGAAGAAACCAGCAG |
132 |
(14) |
|
mecA
|
ACTGCTATCCACCCTCAAAC
CTGGTGAAGTTGTAATCTGG |
163 |
(14) |
(iii) Preparation of Master Mix and Primer with Template DNA
Sterile 0.2 mL microcentrifuge tubes/PCR tubes were taken and labeled with the date and identification number. Then, 15 µLmaster buffer (Texas Bio Gene Inc, Texas, United States) composed of a mixture of PCR buffer, MgCl2, deoxynucleoside triphosphate (dNTP), 0.1 µLof Taq polymerase (Geneaid Biotech Ltd., New Taipei, Taiwan), and 3 µL of sterile distilled water were loaded in each PCR tube. This was followed by adding 0.5 µL of each gene-specific primer into the tubes. The mixture containing the master mix, Taq polymerase, and primers was vortexed and spun for a short time. Next, 2 µL of extracted DNA from each separate sample was added to the tubes. Then, PCR tubes were centrifuged in a microcentrifuge for 5 seconds.
(iv) DNA Amplification in Thermal Cycler
An automated DNA thermal cycler (Applied Biosystem 2720, Life Technologies, California, USA) was used for DNA amplification. After placing the PCR tubes in the thermal cycler, a protocol was run(14) involving initial denaturation at 94°C for 5 minutes, followed by 35 cycles of amplification. Each amplification cycle consisted of denaturation at 94°C for 2 minutes, annealing at 57°C for 2 minutes, and extension at 72°C for 1 minute, and after the completion of 35 cycles, a final extension was done at 72°C for 7 minutes.
(v) Amplicon Detection by Agarose Gel Electrophoresis
The amplified PCR products were detected by electrophoresis in 2% agarose gel with ethidium bromide (0.5 µg/mL) prepared in 1 × Tris acetate-EDTA buffer (Tris Acetic Acid EDTA- Ethylene diamine tetra acetic acid). The prepared gel with the stand was placed in a horizontal electrophoresis tank (Biometra Compact Multi-Wide; Analytik Jena, Jena, Germany) containing 1 × Tris acetate-EDTA buffer. Next, approximately 10 µL of amplified products were loaded into the wells using disposable micropipette tips, and 10 µL of amplified product of negative control was loaded into a different well. Afterward, 7 µL of DNA molecular size marker (100 bp ladder; Texas Bio Gene Inc, Texas, USA) was added in one well to assess the size of amplified PCR products, and electrophoresis was run at 110 V for 90 minutes.
(vi) Visualization and Documentation by UV Trans-illuminator
Following electrophoresis, the visualization of the DNA bands was carried out using an ultra-violet transilluminator (Vilber Lourmat, Eberhardzell, Germany). A digital camera was used to take photos of the gel, and the data were then transferred to a computer for additional documentation. The identification of the bands was done according to their molecular size by comparing them with the DNA molecular size marker (100 bp ladder). Samples were recorded as positive when PCR products of approximately 132 bp in the case of femA gene and 163 bp in the case of mecA gene were observed, and no amplicons were detected for the negative control (Figure 1).
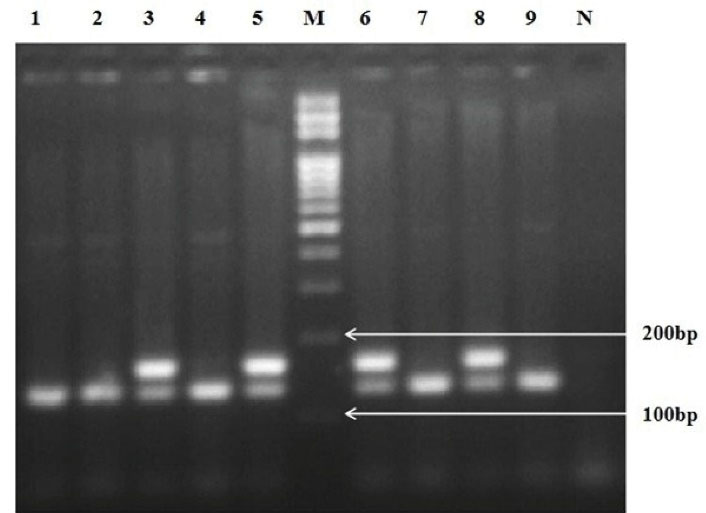
Figure 1.
Agarose Gel Electrophoresis Patterns Showing Amplified DNA Products of 132 bp for femA Gene and 163bp for mecA Gene. Note. Lane M: DNA molecular size marker (100bp ladder; Texas Bio Gene Inc, Texas, USA), Lane N: Negative control. Lane 1: femA, lane 2: femA, lane 3: femA + mecA, lane 4: femA, lane 5: femA + mecA, lane 6: femA + mecA, lane 7: femA, lane 8: femA + mecA, lane 9: femA
.
Agarose Gel Electrophoresis Patterns Showing Amplified DNA Products of 132 bp for femA Gene and 163bp for mecA Gene. Note. Lane M: DNA molecular size marker (100bp ladder; Texas Bio Gene Inc, Texas, USA), Lane N: Negative control. Lane 1: femA, lane 2: femA, lane 3: femA + mecA, lane 4: femA, lane 5: femA + mecA, lane 6: femA + mecA, lane 7: femA, lane 8: femA + mecA, lane 9: femA
Data Analysis
For data analysis, the Statistical Package for the Social Sciences (SPSS) software version 21 (SPSS Inc., Chicago, USA) was used. For comparison of differences in the antibiotic susceptibility data between MRSA and MSSA strains, the P value was calculated by the chi-square test or Fisher’s exact test when applicable, and a P value < 0.05 was regarded to be statistically significant.
Results
The 185 S. aureus isolates included in the study were initially identified by colony characteristics and various biochemical tests, and all (100%) isolates tested positive for the femA gene by PCR assay which confirmed their identity as S. aureus. PCR assay for the mecA gene showed that out of the 185 isolates, 76 (41.1%) were MRSA (positive for the mecA gene), and 109 (58.9%) were MSSA (negative for the mecA gene), as depicted in Table 2. Out of the three tertiary care hospitals, MRSA was detected to be the highest in DMC (49.2%), followed by BSMMU (39%), and BIRDEM (35.8%), as displayed in Table 2.
Table 2.
The Frequency of the Detection of MRSA and MSSA by PCR Assay
|
Name of Tertiary Care Hospital
|
MRSA No. (%)
|
MSSA No. (%)
|
The Number of Collected
Staphylococcus
aureus
Isolates
|
| BSMMU |
16 (39) |
25 (61) |
41 |
| DMC |
31 (49.2) |
32 (50.8) |
63 |
| BIRDEM |
29 (35.8) |
52 (64.2) |
81 |
| Total |
76 (41.1%) |
109 (58.9%) |
185 |
Abbreviations: MRSA, Methicillin resistant S. aureus; MSSA, Methicillin sensitive S. aureus; PCR: Polymerase chain reaction; BSMMU, Bangabandhu Sheikh Mujib Medical university; DMC, Dhaka Medical College; BIRDEM, Bangladesh Institute of Research and Rehabilitation in Diabetes, Endocrine, and Metabolic Disorders.
Regarding clinical specimens, most of the S. aureus (108, 58.4%) were isolated from pus followed by 32 (17.3%) from blood, 31 (16.7%) from wound swabs, and 14 (7.6%) isolates from urine (Table 3).
Table 3.
The Frequency of Staphylococcus aureus Isolation According to Clinical Specimen
|
Clinical Specimen
|
S. aureus
Isolates
No. (%)
|
MRSA
No. (%)
|
MSSA
No. (%)
|
| Pus |
108 (58.4) |
38 (50) |
70 (64.2) |
| Wound Swab |
31 (16.7) |
19 (25) |
12 (11) |
| Blood |
32 (17.3) |
14 (18.4) |
18 (16.5) |
| Urine |
14 (7.6) |
5 (6.6) |
9 (8.3) |
| Total |
185 |
76 |
109 |
Abbreviations: MRSA, Methicillin resistant S. aureus; MSSA, Methicillin sensitive S. aureus.
Table 4 presents the antibiotic susceptibility pattern of the 185 S. aureus isolates, 76 MRSA, and 109 MSSA against 14 antimicrobial agents. All the isolates were sensitive to vancomycin (100%) and linezolid (100%), followed by high levels of sensitivity to rifampicin (94%), meropenem (87%), gentamicin (85.4%), cotrimoxazole (82.2%), cloxacillin (75.1%), ceftriaxone (64.3%), and cefoxitin (61.1%). Regarding the resistance pattern, the majority of isolates were resistant to penicillin G (94.6%), followed by amoxicillin/clavulanic acid (82.7%), azithromycin (72.4%), amoxicillin (66.5%), and ciprofloxacin (63.2%).
Table 4.
Antibiotic Susceptibility Pattern of S. aureus (n = 185), MRSA (n = 76), and MSSA (n = 109) isolates
|
Name of Antibiotic
|
S. aureus
Isolates (n=185)
|
MRSA (n=76)
|
MSSA (n=109)
|
P
Value
|
Sensitive
No. (%)
|
Resistant
No. (%)
|
Sensitive
No. (%)
|
Resistant
No. (%)
|
Sensitive
No. (%)
|
Resistant
No. (%)
|
| Penicillin G |
10 (5.4) |
175 (94.6) |
00 |
76 (100) |
10 (9.2) |
99 (90.8) |
0.003 |
| Cloxacillin |
139 (75.1) |
46 (24.9) |
35 (46.1) |
41 (53.9) |
104 (95.4) |
5 (4.6) |
0.000 |
| Amoxicillin |
62 (33.5) |
123 (66.5) |
12 (15.8) |
64 (84.2) |
50 (45.9) |
59 (54.1) |
0.000 |
| Amoxicillin + Clavulanic acid |
32 (17.3) |
153 (82.7) |
4 (5.3) |
72 (94.7) |
28 (25.7) |
81 (74.3) |
0.000 |
| Ceftriaxone |
119 (64.3) |
66 (35.7) |
16 (21.1) |
60 (78.9) |
103 (94.5) |
6 (5.5) |
0.000 |
| *Cefoxitin |
113 (61.1) |
72 (38.9) |
7 (9.2) |
69 (90.8) |
106 (97.2) |
3 (2.8) |
0.000 |
| Meropenem |
161 (87) |
24 (13) |
55 (72.4) |
21 (27.6) |
106 (97.2) |
3 (2.8) |
0.000 |
| Gentamicin |
158 (85.4) |
27 (14.6) |
51 (67.1) |
25 (32.9) |
107 (98.2) |
2 (1.8) |
0.000 |
| Azithromycin |
51 (27.6) |
134 (72.4) |
13 (17.1) |
63 (82.9) |
38 (34.9) |
71 (65.1) |
0.004 |
| Cotrimoxazole |
152 (82.2) |
33 (17.8) |
57 (75) |
19 (25) |
95 (87.2) |
14 (12.8) |
0.030 |
| Ciprofloxacin |
68 (36.8) |
117 (63.2) |
19 (25) |
57 (75) |
49 (45) |
60 (55) |
0.006 |
| Rifampicin |
174 (94) |
11 (6) |
67 (88.2) |
9 (11.8) |
107 (98.2) |
2 (1.8) |
0.029 |
| Vancomycin |
185(100) |
00 |
76 (100) |
00 |
109 (100) |
00 |
N/A |
| Linezolid |
185 (100) |
00 |
76(100) |
00 |
109 (100) |
00 |
N/A |
Abbreviations: MRSA, Methicillin resistant S. aureus; MSSA, Methicillin sensitive S. aureus; PCR: Polymerase chain reaction; N/A: Not applicable.
*Results of the cefoxitin disc diffusion test.
The results of the MRSA screening by CDDT indicated that 7 (9.2%) out of 76 MRSA (positive formecA gene by PCR) were sensitive to cefoxitin, and 3 (2.8%) out of 109 MSSA (negative formecA gene by PCR) were resistant to cefoxitin (Table 4).
In this study, since all of the MRSA and MSSA were sensitive to vancomycin, and linezolid statistical analysis was not applicable for the two antibiotics, and for the remaining 12 antibiotics tested, MRSA exhibited higher levels of resistance compared to the MSSA, and the results were statistically significant (P < 0.05), as depicted in Table 4.
Moreover, the overall prevalence of MDR S. aureus was 81.1% (150 out of 185 isolates). Among the MRSA, the majority were MDR at 97.4% (74 out of 76 isolates), while 69.7% (76 out of 109 isolates) of the MSSA were MDR. The 2 MRSA isolates (positive formecA gene by PCR) that were not MDR were each resistant to only 2 antimicrobial categories, penicillins, and β- lactamase inhibitors.
Discussion
It is well established that multiplex PCR can be utilized to identify staphylococcal strains and associated methicillin resistance (14,15,24). In this study, all of the 185 clinical S. aureus isolateswere subjected to the duplex PCR assay for the simultaneous detection of the femA and the mecA genes. ThefemA gene was used to confirm the identity of S. aureus and used as an internal positive control for PCR assay; moreover, it was present in all of the 185 isolates (100%). Regarding the detection of the mecA gene by PCR assay,76 (41.1%) were MRSA and 109 (58.9%) were MSSA. This correlates with previous studies from Bangladesh by Haque et al and Dutta et al in which MRSA prevalence was 43.5% and 46.0%, respectively (25,26). Similar findings were also reported from India (43.6%), Pakistan (45.3%), and Cameroon (45.5%), respectively (27-29).
Among the three institutions, the MRSA isolation rate was detected to be the highest at 49.2% in DMC, followed by 39% in BSMMU and 35.8% in BIRDEM. Our findings are in concordance with the multicentre study from Bangladesh by Haq et al, which reported that the isolation rate of MRSA among five hospitals ranges between 32.1% and 63.0%. Their study found that the incidence of MRSA was high in the hospitals investigated from different parts of Bangladesh (30). The reason for the high prevalence of MRSA in our study might be due to the fact the study isolates were taken from tertiary care hospitals where patients are at risk for MRSA infections, particularly the elderly, immunocompromised and intensive care unit patients, burn patients, and patients with surgical wounds and intravenous lines. Further contributing factors are the duration of hospitalization, previous antibiotic treatment, person-to-person transmission of MRSA generally via the hands of healthcare workers, and the proximity to other patients colonized or infected with MRSA (31). Compared to the present study, MRSA prevalence was observed at higher levels in studies from Iraq (73.2%) and Saudi Arabia (82%), respectively (32,33), whereas lower MRSA detection rates were reported from France (6%), Ireland (5%), and United Kingdom (1.8%) (34).
Geographic variations in the prevalence of MRSA between countries and variations between different medical institutions within a given regional area may be attributed to several factors such as the efficacy of infection control practices, the rationale use of antibiotics in healthcare facilities which varies from one hospital to another, population variations, differences in microbiological methods, and differences in the biological characteristics of the S. aureus strains in the region in terms of the clone and its epidemic nature (29,35-37).
In this study, the highest frequency of S. aureus (58.4%), MRSA (50%), and MSSA (64.2%) was isolated from pus specimens. The findings of this study are in agreement with studies from Pakistan, Cameroon, Nepal and India, which found the highest numbers of isolates recovered from pus specimens (28,29,37,38). This highlights the crucial role of S. aureus in the formation of abscesses and the pyogenic nature of the infections (19,28,39). The frequency of S. aureus (17.3%) and MRSA (18.4%) bacteraemia in this study was compatible with studies conducted in Pakistan (18.9% of S. aureus), Nepal (17.1% of S. aureus and 15.8% of MRSA) and Nigeria (18.7% of S. aureus) (28,37,40). In contrast, low levels of bacteraemia have been reported by Iileka et al (19) from Nigeria (4.2% of S. aureus). Compared with the wound swab isolation levels of our study (16.7% of S. aureus and 25% of MRSA), most researchers reported higher levels (26,40,41). The frequency of isolates from urine specimens was the lowest in this study (7.6% of S. aureus and 6.6% of MRSA) and similar to the figures in studies from Nigeria (6.7% of S. aureus) and Jordan (3.6% of S. aureus and 2.7% of MRSA) (40,41); however, higher values were observed by Rashmi et al (38) from India (30.9% of S. aureus and 31% of MRSA). These results illustrate that the type of S. aureus infections differs between different countries. These variations may be related to many reasons such as the patient population, types of skin normal flora, collection procedures, and the number of specimens (41).
Cefoxitin is widely used as a marker for the detection of mecA gene-mediated methicillin resistance because it is a strong inducer of the mecA regulatory system (11,27,42). In this study, the CDDT found 7 (9.2%) out of the 76 MRSA (mecA positive) strains sensitive to cefoxitin. This sort of disparity was also found in studies by Bhutia et al (27) and Jain et al (43). The reason underlying this is that the phenotypic methods depend on various factors such as the growth conditions (e.g., temperature, incubation period, inoculum size, osmolarity, pH of the medium, culture medium supplements such as sodium chloride concentration, and reading of endpoints) which have a significant effect on the expression and therefore the detection of resistance. It also depends on the over-expression of mecR and mecI genes which are co-repressors of mecA gene, the carriage of a non-functional or a non-expressed mecA gene which is not expressed unless selective pressure via antibiotic treatment is applied. Moreover, most MRSA strains express heterogeneous resistance, where large differences in the degree of resistance to β-lactam agents exist among individual cells in a population and the level of resistance expressed depends on the varying testing conditions and the type of β-lactam antibiotic used and this limits the accuracy and reliability of phenotypic methods like CDDT, however genotypic methods are not influenced by such factors (12,13,27,44-47). Therefore, mecA gene detection, recognized as the gold standard method, is advocated for the precise identification of MRSA, and PCR can be used as a standard method in clinical laboratories for the comparison of phenotypic testing (12,13,42).
Among the 109 MSSA (mecA negative) strains, 3 (2.8%) were resistant to cefoxitin by CDDT. This type of discordant result was also reported in previous studies (27,43), while other studies found no discrepancy between CDDT and PCR assay for the mecA gene (42,48). The reason for the MSSA strains expressing resistance phenotypically may be attributed to the hyperproduction of β-lactamases causing partial hydrolysis of the beta-lactam ring resulting in the phenotypic expression of resistance. Such strains are referred to as borderline oxacillin-resistant S. aureus. Another reason may be due to modified S. aureus that has modifications of the PBP, leading to low affinity (27,42,49). Research has also shown that the PBP4 gene can express resistance in MRSA in addition to the mecA gene (50).
Moreover, recent studies from several European countries have reported the detection of a rare gene homologous to mecA in S. aureus, isolated from human and animal MRSA strains. This gene was originally called mecALGA251 and is now designated as mecC, which is encoded in a novel staphylococcal cassette chromosome mec element identified as type XI (51,52). The mecC gene exhibits phenotypic resistance to methicillin in disc diffusion tests with cefoxitin, but it cannot be detected by standard mecA gene-specific PCR methods or PBP2a slide agglutination tests. Therefore, the mecC gene is a potential diagnostic dilemma and raises a number of questions for future research (51-53). Despite the rarity of mecC gene detection, such observations might be another possible explanation for our findings. From the viewpoint of clinical practice, this type of discrepancies among test results suggests caution when using a single method for identifying methicillin resistance in S. aureus because it can result in inaccurate results with serious potential consequences for individual patients receiving inappropriate medications and for the surveillance of MRSA (53,54).
In antibiogram, all S. aureus isolates in our study were 100% sensitive to vancomycin and linezolid, and our results are comparable to studies from different countries, which found full susceptibility to these antibiotics in both MRSA and MSSA isolates (6,33,55). This suggests that they might be suitable treatment options when indicated. Vancomycin continues to be the antibiotic of choice for the treatment of MDR MRSA strains causing life-threatening infections (6,32,37,56), and linezolid is effective against both MRSA and strains resistant to glycopeptides (57). The majority of S. aureus remains susceptible probably due to the unique mechanism of action of these antibiotics, which makes it difficult to develop resistance easily. Vancomycin inhibits the ribonucleic acid synthesis and cell wall synthesis, and it has lethal membrane effects; further, the activity at these three sites accounts for the lack of development of resistance to vancomycin readily (56). The mechanism of action of linezolid is via inhibiting protein synthesis before the formation of the initiation complex, and this is distinct from other known protein synthesis inhibitors (13).
Despite this, several reports have documented the prevalence of vancomycin-resistant S. aureus and vancomycin-intermediate S. aureus in Bangladesh, Ethiopia, Uganda, United Kingdom, and Japan with a background of MRSA infections (48,58-61), and a small number of S. aureus resistant to linezolid were reported by other studies (62,63). Therefore, when vancomycin or linezolid is considered for treatment, it inevitably requires routine testing of every isolate of MRSA (37). Additionally, regular monitoring should be done to prevent the rapid emergence of resistance.
Rifampicin was the second most sensitive drug in which 94% S. aureus, 88.2% MRSA, and 98.2% MSSA isolates were susceptible. Studies from Jordan (92.7% S. aureus and 85.8% MRSA), Korea (82% MRSA and 99.2% MSSA), and Pakistan (95.7% S. aureus) reported rifampicin sensitivity levels that concur with the data in the present study (41,55,62). Rifampicin is an extremely potent bactericidal anti-staphylococcal agent that penetrates well into tissues and abscesses, exhibiting the treatment of serious invasive staphylococcal infections (13,64). However, lower levels of sensitivity to rifampicin have been reported by some investigators (6,65). Resistance develops if it is used as monotherapy; therefore, rifampicin should be used only in combination with another anti-staphylococcal agent to which the isolate is also susceptible (3,13).
Overall 85.4% of S. aureus was sensitive to gentamicin, and similar high percentages were reported by researchers from Iraq (95.7%) and Bangladesh (92.5%), respectively (32,66). Although almost all MSSA (98.2%, 107 out of 109) were susceptible to gentamicin, 67.1% of MRSA were found sensitive. In Bangladesh, gentamicin is not commonly prescribed for S. aureus infections, so the resistance is low, showing that this drug might be a good choice for treatment when indicated. Due to their synergistic activity, increased bactericidal action, and ability to prevent the development of resistance, gentamicin and other aminoglycosides are frequently used in combination with anti-staphylococcal penicillin or vancomycin to treat endocarditis and other life-threatening invasive staphylococcal infections (3,13,64).
Cotrimoxazole susceptibility was also reasonably good in our study in which 82.2% S. aureus, 75% MRSA, and 87.2% MSSA were found sensitive. Cotrimoxazole is an older drug and is no longer in common use in our region due to its side effects, and this may be a possible reason for the gradual increase in efficacy. Our results are in accordance with Al Zoubi et al (83.2% S. aureus) and Kejela and Bacha (94.7% S. aureus, 82.1% MRSA) where they found high levels of cotrimoxazole sensitivity (41,67). Cotrimoxazole could be a viable alternative for treating minor to moderately severe S. aureus infections, particularly skin and soft tissue infections caused by MSSA and MRSA (3,64).
Moreover, the resistance to ciprofloxacin was moderately high in the present study, involving more than half of the study isolates (63.2% S. aureus, 75% MRSA, and 55% MSSA). This is in accordance with the findings of several previous studies from Bangladesh that reported ciprofloxacin-resistant S. aureus ranging from 51.7% to 69% (26,48,66,68). In another study from Bangladesh, Shamsuzzaman et al documented the increase in resistance of S. aureus against ciprofloxacin from 17% to 43% during the years 2001 to 2003 (18). The study also mentioned that ciprofloxacin is frequently used empirically and improperly in both community and medical settings of the country and that the extended and irrational exposure to the antibiotic may cause genomic modifications among the bacterial strains of the region (18), which may be an explanation for our findings. Furthermore, both national and international studies have reported a higher incidence of resistance among MRSA which coincides with our data (6,48,59,65). On the other hand, studies from some African countries reported no resistance and extremely low levels of resistance to fluoroquinolones and ciprofloxacin, which were attributed to the fact that S. aureus clones in those regions have not yet been exposed to extensive use; therefore, they have not developed mechanisms of resistance (19,29). Due to the significant levels of resistance found in our investigation, the blind use of ciprofloxacin for curative purposes or quinolone-based combination therapy might not be practical in the study area.
Poor susceptibility was also observed towards azithromycin with 72.4% S. aureus isolates resistant (82.9% MRSA and 65.1% MSSA). However, most of the studies reported a moderate resistance of S. aureus to azithromycin ranging from 38.1% to 56.3%, which was lower compared to the data obtained in the present study (32,48,66,68). The finding concerning high azithromycin resistance in MRSA (82.9%) in the present study is in line with the works from Bangladesh by Islam and Shamsuzzaman and Ahmed et al which reported 73.3% and 78% resistance in MRSA, respectively (48,69). Azithromycin and more recent macrolide derivatives are effective against a broader spectrum of pathogenic bacteria, and their pharmacokinetic properties allow for less frequent dosing compared to erythromycin; as a result, a dramatically increased usage has resulted in greater exposure of bacterial populations to macrolides and emergence of resistance (70) which may explain the findings of our study.
Furthermore, penicillin G showed the highest overall resistance in our study among all the S. aureus isolates (94.6%) in both MRSA (100%) and MSSA (90.8%). These results are in agreement with previous studies from Bangladesh and abroad which reported over 90% resistance to penicillin (19,26,28,30,32,37,38,58,71). This can be explained by the fact that only a small percentage of the S. aureus lineages lack the genes necessary to produce β-lactamase (39).
The resistance to the other β-lactam antibiotics tested in this study, including cloxacillin, ceftriaxone, amoxicillin, cefoxitin, and amoxicillin/clavulanic acid was also high in MRSA ranging from 53.9% to 94.7% except for meropenem (27.6%); however, the reason for low resistance to meropenem could not be found. Similarly, other investigators from Bangladesh (69) and Iraq (32,72) also found high resistance of MRSA against the β-lactams except for carbapenems in their studies. The emergence of antibiotic-resistant bacteria reflects the intensive use of antibiotic agents (73), and β-lactams may cause a selective pressure for the selection and emergence of mutant strains expressing homogeneous resistance to β-lactams from heterogeneous strains (72). The most clinically significant resistance mechanism is the acquisition of mecA gene, which is intrinsically resistant to inhibition by β- lactams (46), and another reason is the production of β-lactamase (penicillinase) enzyme that breaks open the β-lactam ring of the antibiotics, rendering them ineffective (39,71).
MSSA isolates on the other hand showed high levels of sensitivity to meropenem (97.2%), cefoxitin (97.2%), cloxacillin (95.4%), and ceftriaxone (94.5%), making these β-lactams suitable options for the treatment of MSSA infections in the study area. However, poor sensitivity to amoxicillin (45.9%) and amoxicillin/clavulanic acid combination (25.7%) was observed. Similarly, other researchers have reported the reduced efficacy of these two β-lactam antibiotics (26,62,66). A reasonable explanation for this might be that amoxicillin and amoxicillin/clavulanic acid combination are among the commonly used β-lactam agents in our region, and their intensive use may have enhanced the resistance in MSSA isolates as well.
In this study, MRSA isolates were significantly more resistant to antibiotics compared to MSSA. A significant difference (P value < 0.05) was documented against all the antibiotics tested, except for vancomycin and linezolid (statistical analysis was not applicable as all isolates were susceptible). Likewise, previous studies have revealed a relationship between methicillin resistance and resistance to other antibiotics (33,37,55,58,67). Cross-resistance of MRSA against different antibiotics in addition to β-lactam resistance is due to the mecA gene and its accompanying DNA functioning as a region for the integration of various determinants, including genes for drug resistance (7,13).
The overall prevalence of MDR isolates in the present study was 81.1% in S. aureus, 97.4% in MRSA, and 69.7% in MSSA. Moreover, high levels of MDR have been reported in S. aureus by Siddiqui et al (68%) and in MRSA isolates by Fluit et al (87%) and Pandey et al (75.9%), but their figures were lower compared to our data (6,37,74). The study by Kim et al found that the majority of MRSA (97.7%, 429 out of 439 isolates) were MDR, which is similar to our findings (55). Elevated rates of MDR generally emerge from diverse isolates of S. aureus under antimicrobial pressure caused by the intensive use of topical and systemic antimicrobial agents, which produce a highly selective pressure for the antibiotic-resistant bacterial clones or due to widespread person-to-person transmission of MDR isolates (55,73).
Conclusion
In summary, the findings of this study showed a high prevalence of MRSA among the clinical S. aureus isolates from tertiary care hospitals in this region. Among the studied clinical specimens, the highest frequency of both MRSA and MSSA was isolated from pus and the least from urine. All the S. aureus isolates, including MRSA, were sensitive to vancomycin and linezolid, and the majority was susceptible to rifampicin, gentamicin, and cotrimoxazole; in addition, the MSSA exhibited high sensitivity to meropenem, cefoxitin, cloxacillin, and ceftriaxone, making them suitable options for therapy when indicated. MRSA was significantly more resistant to antibiotics than MSSA, and the prevalence of MDR was alarmingly high, detected in almost all MRSA and a considerable percentage of MSSA.
The information obtained from this study could serve as a guideline for physicians in the region to select the appropriate antibiotic for empirical and treatment purposes. The study also highlights the need to formulate and adhere to definite antibiotic policy, conduct regular surveillance studies, track emerging drug-resistance patterns, and implement effective infection prevention and control practices to combat antibiotic resistance and curb the spread of MRSA and MDR in S. aureus in the environment.
Acknowledgements
We are grateful to the Microbiology Departments of BSMMU, DMC, and BIRDEM for their cooperation during the study. This research work was funded by the Research Grant for Students of BSMMU.
Authors’ Contribution
Conceptualization: Fatema Mohammad Alam.
Data collection: Fatema Mohammad Alam, Aysha Khatun.
Formal Analysis: Fatema Mohammad Alam, Ahmed Abu Saleh.
Funding acquisition: Fatema Mohammad Alam.
Investigation: Fatema Mohammad Alam.
Methodology: Fatema Mohammad Alam, Aysha Khatun.
Project administration: Fatema Mohammad Alam, Ahmed Abu Saleh.
Resources: Fatema Mohammad Alam, Ahmed Abu Saleh.
Software: Fatema Mohammad Alam, Abdur Rahman Mohammad Alam, Nabila Afroze.
Supervision: Fatema Mohammad Alam, Ahmed Abu Saleh, Sanjida Khondakar Setu.
Validation: Fatema Mohammad Alam, Tamanna Tasnim, Sonia Afroz, Abdur Rahman Mohammad Alam, Nabila Afroze, Sanjida Khondakar Setu, Ahmed Abu Saleh.
Writing the original draft: Fatema Mohammad Alam.
Competing Interests
There is no conflict of interests as stated by the authors.
Ethical Approval
Ethical approval for this study was granted by the Institutional Review Board of BSMMU with process number: BSMMU/2016/8045, and informed consent was not required for this study.
References
- Sampathkumar P. Methicillin-resistant Staphylococcus aureus: the latest health scare. Mayo Clin Proc 2007; 82(12):1463-7. doi: 10.1016/s0025-6196(11)61088-4 [Crossref] [ Google Scholar]
- Lowy FD. Staphylococcus aureus infections. N Engl J Med 1998; 339(8):520-32. doi: 10.1056/nejm199808203390806 [Crossref] [ Google Scholar]
- Pantosti A, Sanchini A, Monaco M. Mechanisms of antibiotic resistance in Staphylococcus aureus. Future Microbiol 2007; 2(3):323-34. doi: 10.2217/17460913.2.3.323 [Crossref] [ Google Scholar]
- Patel JB. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fourth Informational Supplement. 24th ed. Clinical & Laboratory Standards Institute; 2014.
- Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012; 18(3):268-81. doi: 10.1111/j.1469-0691.2011.03570.x [Crossref] [ Google Scholar]
- Fluit AC, Wielders CL, Verhoef J, Schmitz FJ. Epidemiology and susceptibility of 3,051 Staphylococcus aureus isolates from 25 university hospitals participating in the European SENTRY study. J Clin Microbiol 2001; 39(10):3727-32. doi: 10.1128/jcm.39.10.3727-3732.2001 [Crossref] [ Google Scholar]
- Hiramatsu K, Katayama Y, Matsuo M, Sasaki T, Morimoto Y, Sekiguchi A. Multi-drug-resistant Staphylococcus aureus and future chemotherapy. J Infect Chemother 2014; 20(10):593-601. doi: 10.1016/j.jiac.2014.08.001 [Crossref] [ Google Scholar]
- Yusuf MA, Sattar AA, Habib ZH, Roy S. Clinical and diagnostic significance of methicillin-resistant Staphylococcus aureus infection in Bangladesh: a systematic review. Br Microbiol Res J 2014; 4(7):785-97. doi: 10.9734/bmrj/2014/8071 [Crossref] [ Google Scholar]
- Lowy FD. Antimicrobial resistance: the example of Staphylococcus aureus. J Clin Invest 2003; 111(9):1265-73. doi: 10.1172/jci18535 [Crossref] [ Google Scholar]
- Unal S, Hoskins J, Flokowitsch JE, Wu CY, Preston DA, Skatrud PL. Detection of methicillin-resistant staphylococci by using the polymerase chain reaction. J Clin Microbiol 1992; 30(7):1685-91. doi: 10.1128/jcm.30.7.1685-1691.1992 [Crossref] [ Google Scholar]
- Fernandes CJ, Fernandes LA, Collignon P. Cefoxitin resistance as a surrogate marker for the detection of methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother 2005; 55(4):506-10. doi: 10.1093/jac/dki052 [Crossref] [ Google Scholar]
- Brown DF. Detection of methicillin/oxacillin resistance in staphylococci. J Antimicrob Chemother 2001; 48 Suppl 1:65-70. doi: 10.1093/jac/48.suppl_1.65 [Crossref] [ Google Scholar]
- Chambers HF. Methicillin resistance in staphylococci: molecular and biochemical basis and clinical implications. Clin Microbiol Rev 1997; 10(4):781-91. doi: 10.1128/cmr.10.4.781 [Crossref] [ Google Scholar]
- Mehrotra M, Wang G, Johnson WM. Multiplex PCR for detection of genes for Staphylococcus aureus enterotoxins, exfoliative toxins, toxic shock syndrome toxin 1, and methicillin resistance. J Clin Microbiol 2000; 38(3):1032-5. doi: 10.1128/jcm.38.3.1032-1035.2000 [Crossref] [ Google Scholar]
- Vannuffel P, Gigi J, Ezzedine H, Vandercam B, Delmee M, Wauters G. Specific detection of methicillin-resistant Staphylococcus species by multiplex PCR. J Clin Microbiol 1995; 33(11):2864-7. doi: 10.1128/jcm.33.11.2864-2867.1995 [Crossref] [ Google Scholar]
- Rossolini GM, Arena F, Pecile P, Pollini S. Update on the antibiotic resistance crisis. Curr Opin Pharmacol 2014; 18:56-60. doi: 10.1016/j.coph.2014.09.006 [Crossref] [ Google Scholar]
- Jorgensen JH. Selection criteria for an antimicrobial susceptibility testing system. J Clin Microbiol 1993; 31(11):2841-4. doi: 10.1128/jcm.31.11.2841-2844.1993 [Crossref] [ Google Scholar]
- Shamsuzzaman AK, Paul SK, Mahmud MC, Musa AK, Hossain MA. Emerging antimicrobial resistance amongst common bacterial pathogens in Mymensingh Medical College Hospital. Bangladesh J Med Microbiol 2007; 1(1):4-9. doi: 10.3329/bjmm.v1i1.20488 [Crossref] [ Google Scholar]
- Iileka AE, Mukesi M, Engelbrecht F, Moyo SR. Antimicrobial susceptibility patterns of Staphylococcus aureus strains isolated at the Namibia Institute of Pathology from 2012 to 2014. Open J Med Microbiol 2016; 6(3):116-24. doi: 10.4236/ojmm.2016.63016 [Crossref] [ Google Scholar]
- Sutter DE, Milburn E, Chukwuma U, Dzialowy N, Maranich AM, Hospenthal DR. Changing susceptibility of Staphylococcus aureus in a US pediatric population. Pediatrics 2016; 137(4):e20153099. doi: 10.1542/peds.2015-3099 [Crossref] [ Google Scholar]
- El-Ghodban A, Ghenghesh KS, Márialigeti K, Esahli H, Tawil A. PCR detection of toxic shock syndrome toxin of Staphylococcus aureus from Tripoli, Libya. J Med Microbiol 2006; 55(Pt 2):179-82. doi: 10.1099/jmm.0.46162-0 [Crossref] [ Google Scholar]
- Collee JG, Miles RS, Watt B. Tests for the identification of bacteria. In: Collee JG, Fraser AG, Marmion BP, Simmons A, eds. Mackie & McCartney Practical Medical Microbiology. 14th ed. New York: Churchill Livingstone; 1996. p. 131-49.
- Cheesbrough M. Microscopical techniques used in microbiology, culturing bacterial pathogens, biochemical tests to identify bacteria. In: Cheesbrough M, ed. District Laboratory Practice in Tropical Countries, Part 2. 2nd ed. Edinburgh, UK: Cambridge University Press; 2006. p. 35-70.
- Strommenger B, Kettlitz C, Werner G, Witte W. Multiplex PCR assay for simultaneous detection of nine clinically relevant antibiotic resistance genes in Staphylococcus aureus. J Clin Microbiol 2003; 41(9):4089-94. doi: 10.1128/jcm.41.9.4089-4094.2003 [Crossref] [ Google Scholar]
- Haque ME, Shahriar M, Haq A, Gomes BC, Hossain MM, Razzak MA. Prevalence of β-lactamase-producing and non-producing methicillin resistant Staphylococcus aureus in clinical samples in Bangladesh. Journal of Microbiology and Antimicrobials 2011; 3(5):112-8. [ Google Scholar]
- Dutta S, Hassan MR, Rahman F, Jilani MS, Noor R. Study of antimicrobial susceptibility of clinically significant microorganisms isolated from selected areas of Dhaka, Bangladesh. Bangladesh J Med Sci 2013; 12(1):34-42. doi: 10.3329/bjms.v12i1.13351 [Crossref] [ Google Scholar]
- Bhutia KO, Singh TS, Biswas S, Adhikari L. Evaluation of phenotypic with genotypic methods for species identification and detection of methicillin resistant in Staphylococcus aureus. Int J Appl Basic Med Res 2012; 2(2):84-91. doi: 10.4103/2229-516x.106348 [Crossref] [ Google Scholar]
- Ravesh-Barakzai Z, Arshad-Khan J, Hussain S. Current antibiotic resistance trend in clinical isolates of Staphylococcus aureus from a tertiary care hospital. J Med Bacteriol 2013; 2(3-4):47-55. [ Google Scholar]
- Bissong ME, Wirgham T, Enekegbe MA, Niba PT, Foka FE. Prevalence and antibiotic susceptibility patterns of methicillin resistant Staphylococcus aureus in patients attending the Laquintinie Hospital Douala, Cameroon. Eur J Clin Bioimed Sci 2016; 2(6):92-6. [ Google Scholar]
- Haq JA, Rahman MM, Asna SM, Hossain MA, Ahmed I, Haq T. Methicillin-resistant Staphylococcus aureus in Bangladesh--a multicentre study. Int J Antimicrob Agents 2005; 25(3):276-7. doi: 10.1016/j.ijantimicag.2005.01.004 [Crossref] [ Google Scholar]
- Schmitz FJ, Jones ME. Antibiotics for treatment of infections caused by MRSA and elimination of MRSA carriage. What are the choices? Int J Antimicrob Agents 1997; 9(1):1-19. doi: 10.1016/s0924-8579(97)00027-7 [Crossref] [ Google Scholar]
- Al-Mayahie SM, Al-Hamashee HT, Hameed HM. Prevalence and antimicrobial susceptibility of methicillin-resistant Staphylococcus aureus (MRSA) from outpatients with chronic rhinosinusitis in Al-Kut/Wasit province/Iraq. J Bacteriol Parasitol 2015; 6(3):230. doi: 10.4172/2155-9597.1000230 [Crossref] [ Google Scholar]
- Abbadi S, Youssef H, Nemenqani D, Abdel-Moneim AS. Rapid identification of methicillin resistant Staphylococcus aureus using real time PCR. Adv Infect Dis 2013; 3(1):44-9. doi: 10.4236/aid.2013.31005 [Crossref] [ Google Scholar]
- Denton M, O’Connell B, Bernard P, Jarlier V, Williams Z, Henriksen AS. The EPISA study: antimicrobial susceptibility of Staphylococcus aureus causing primary or secondary skin and soft tissue infections in the community in France, the UK and Ireland. J Antimicrob Chemother 2008; 61(3):586-8. doi: 10.1093/jac/dkm531 [Crossref] [ Google Scholar]
- Kadariya J, Thapaliya D, Bhatta S, Mahatara RL, Bempah S, Dhakal N. Multidrug-resistant Staphylococcus aureus colonization in healthy adults is more common in Bhutanese refugees in Nepal than those resettled in Ohio. Biomed Res Int 2019; 2019:5739247. doi: 10.1155/2019/5739247 [Crossref] [ Google Scholar]
- Baiu SH, AL-Abdli NE. Screening of MRSA in and outside Benghazi hospitals. Am J Microbiol Res 2015; 3(4):144-7. [ Google Scholar]
- Pandey S, Raza MS, Bhatta CP. Prevalence and antibiotic sensitivity pattern of methicillin-resistant-Staphylococcus aureus in Kathmandu Medical College-Teaching Hospital. J Inst Med Nepal 2012; 34(1):13-7. doi: 10.3126/jiom.v34i1.9117 [Crossref] [ Google Scholar]
- Rashmi MS, Krishna S, Qayoom S. Prevalence of MRSA among clinical isolates of Staphylococcus aureus and its antibiotic susceptibility pattern at a tertiary care hospital. Int J Curr Microbiol Appl Sci 2017; 6:747-9. [ Google Scholar]
- Ansari S, Nepal HP, Gautam R, Rayamajhi N, Shrestha S, Upadhyay G. Threat of drug resistant Staphylococcus aureus to health in Nepal. BMC Infect Dis 2014; 14:157. doi: 10.1186/1471-2334-14-157 [Crossref] [ Google Scholar]
- Nwankwo EO, Nasiru MS. Antibiotic sensitivity pattern of Staphylococcus aureus from clinical isolates in a tertiary health institution in Kano, Northwestern Nigeria. Pan Afr Med J 2011; 8:4. doi: 10.4314/pamj.v8i1.71050 [Crossref] [ Google Scholar]
- Al-Zoubi MS, Al-Tayyar IA, Hussein E, Jabali AA, Khudairat S. Antimicrobial susceptibility pattern of Staphylococcus aureus isolated from clinical specimens in Northern area of Jordan. Iran J Microbiol 2015; 7(5):265-72. [ Google Scholar]
- Mathews AA, Thomas M, Appalaraju B, Jayalakshmi J. Evaluation and comparison of tests to detect methicillin resistant S aureus. Indian J Pathol Microbiol 2010; 53(1):79-82. doi: 10.4103/0377-4929.59189 [Crossref] [ Google Scholar]
- Jain A, Agarwal A, Verma RK. Cefoxitin disc diffusion test for detection of meticillin-resistant staphylococci. J Med Microbiol 2008; 57(Pt 8):957-61. doi: 10.1099/jmm.0.47152-0 [Crossref] [ Google Scholar]
- Al-Akydy AG, Daoud HE, Mulhem MM. Disc diffusion method versus PCR for mecA gene in detection of oxacillin resistant Staphylococcus aureus in university children’s hospital in Damascus, Syria. Int J Pharm Pharm Sci 2014; 6(4):488-91. [ Google Scholar]
- Pillai MM, Latha R, Sarkar G. Detection of methicillin resistance in Staphylococcus aureus by polymerase chain reaction and conventional methods: a comparative study. J Lab Physicians 2012; 4(2):83-8. doi: 10.4103/0974-2727.105587 [Crossref] [ Google Scholar]
- Brown DF, Edwards DI, Hawkey PM, Morrison D, Ridgway GL, Towner KJ. Guidelines for the laboratory diagnosis and susceptibility testing of methicillin-resistant Staphylococcus aureus (MRSA). J Antimicrob Chemother 2005; 56(6):1000-18. doi: 10.1093/jac/dki372 [Crossref] [ Google Scholar]
- Martineau F, Picard FJ, Lansac N, Ménard C, Roy PH, Ouellette M. Correlation between the resistance genotype determined by multiplex PCR assays and the antibiotic susceptibility patterns of Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob Agents Chemother 2000; 44(2):231-8. doi: 10.1128/aac.44.2.231-238.2000 [Crossref] [ Google Scholar]
- Islam TAB, Shamsuzzaman SM. Prevalence and antimicrobial susceptibility pattern of methicillin-resistant, vancomycin-resistant, and Panton-Valentine leukocidin positive Staphylococcus aureus in a tertiary care hospital Dhaka, Bangladesh. Tzu Chi Med J 2015; 27(1):10-4. doi: 10.1016/j.tcmj.2014.12.001 [Crossref] [ Google Scholar]
- Tomasz A, Drugeon HB, de Lencastre HM, Jabes D, McDougall L, Bille J. New mechanism for methicillin resistance in Staphylococcus aureus: clinical isolates that lack the PBP 2a gene and contain normal penicillin-binding proteins with modified penicillin-binding capacity. Antimicrob Agents Chemother 1989; 33(11):1869-74. doi: 10.1128/aac.33.11.1869 [Crossref] [ Google Scholar]
- Memmi G, Filipe SR, Pinho MG, Fu Z, Cheung A. Staphylococcus aureus PBP4 is essential for beta-lactam resistance in community-acquired methicillin-resistant strains. Antimicrob Agents Chemother 2008; 52(11):3955-66. doi: 10.1128/aac.00049-08 [Crossref] [ Google Scholar]
- Paterson GK, Morgan FJ, Harrison EM, Cartwright EJ, Török ME, Zadoks RN. Prevalence and characterization of human mecC methicillin-resistant Staphylococcus aureus isolates in England. J Antimicrob Chemother 2014; 69(4):907-10. doi: 10.1093/jac/dkt462 [Crossref] [ Google Scholar]
- Shore AC, Deasy EC, Slickers P, Brennan G, O’Connell B, Monecke S. Detection of staphylococcal cassette chromosome mec type XI carrying highly divergent mecA, mecI, mecR1, blaZ, and ccr genes in human clinical isolates of clonal complex 130 methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 2011; 55(8):3765-73. doi: 10.1128/aac.00187-11 [Crossref] [ Google Scholar]
- Paterson GK, Harrison EM, Holmes MA. The emergence of mecC methicillin-resistant Staphylococcus aureus. Trends Microbiol 2014; 22(1):42-7. doi: 10.1016/j.tim.2013.11.003 [Crossref] [ Google Scholar]
- Nwokah EG, Abbey SD, Wachukwu CK. mecA gene profile of methicillin-resistant Staphylococcus aureus isolates from clinical sources in Port Harcourt, Nigeria. Am J Biomed Life Sci 2016; 4(3):41-8. [ Google Scholar]
- Kim HB, Jang HC, Nam HJ, Lee YS, Kim BS, Park WB. In vitro activities of 28 antimicrobial agents against Staphylococcus aureus isolates from tertiary-care hospitals in Korea: a nationwide survey. Antimicrob Agents Chemother 2004; 48(4):1124-7. doi: 10.1128/aac.48.4.1124-1127.2004 [Crossref] [ Google Scholar]
- Chambers HF. Methicillin-resistant staphylococci. Clin Microbiol Rev 1988; 1(2):173-86. doi: 10.1128/cmr.1.2.173 [Crossref] [ Google Scholar]
- Perry CM, Jarvis B. Linezolid: a review of its use in the management of serious gram-positive infections. Drugs 2001; 61(4):525-51. doi: 10.2165/00003495-200161040-00008 [Crossref] [ Google Scholar]
- Dilnessa T, Bitew A. Antimicrobial susceptibility pattern of Staphylococcus aureus with emphasize on methicilin resistance with patients postoperative and wound infections at Yekatit 12 Hospital Medical College in Ethiopia. Am J Clin Exp Med 2016; 4(1):7-12. [ Google Scholar]
- Nalwoga J, Tirwomwe M, Onchweri AN, Maniga JN, Nyaribo CM, Miruka CO. Drug resistant Staphylococcus aureus in clinical samples at Kampala International University-Teaching Hospital, Bushenyi district, Uganda. Am J Biomed Res 2016; 4(4):94-8. [ Google Scholar]
- Howe RA, Bowker KE, Walsh TR, Feest TG, MacGowan AP. Vancomycin-resistant Staphylococcus aureus. Lancet 1998; 351(9102):602. doi: 10.1016/s0140-6736(05)78597-4 [Crossref] [ Google Scholar]
- Hiramatsu K, Hanaki H, Ino T, Yabuta K, Oguri T, Tenover FC. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J Antimicrob Chemother 1997; 40(1):135-6. doi: 10.1093/jac/40.1.135 [Crossref] [ Google Scholar]
- Mir F, Rashid A, Farooq M, Irfan M, Ijaz A. Antibiotic sensitivity patterns of staphylococcal skin infections. J Pak Assoc Dermatol 2015; 25(1):12-7. [ Google Scholar]
- Wilson P, Andrews JA, Charlesworth R, Walesby R, Singer M, Farrell DJ. Linezolid resistance in clinical isolates of Staphylococcus aureus. J Antimicrob Chemother 2003; 51(1):186-8. doi: 10.1093/jac/dkg104 [Crossref] [ Google Scholar]
- Bal AM, Gould IM. Antibiotic resistance in Staphylococcus aureus and its relevance in therapy. Expert Opin Pharmacother 2005; 6(13):2257-69. doi: 10.1517/14656566.6.13.2257 [Crossref] [ Google Scholar]
- Akoru C, Kuremu RT, Ndege SK, Obala A, Smith JW, Bartlett M. Prevalence and anti-microbial susceptibility of methicillin resistant Staphylococcus aureus at Moi Teaching and Referral Hospital Eldoret. Open J Med Microbiol 2016; 6(1):9-16. [ Google Scholar]
- Islam MS, Ahmed MF, Rahman SR. Incidence of methicillin resistant Staphylococcus aureus in burn patients admitted to burn unit, Dhaka Medical College Hospital, Bangladesh. Adv Microbiol 2013; 3(6):498-503. doi: 10.4236/aim.2013.36066 [Crossref] [ Google Scholar]
- Kejela T, Bacha K. Prevalence and antibiotic susceptibility pattern of methicillin-resistant Staphylococcus aureus (MRSA) among primary school children and prisoners in Jimma town, Southwest Ethiopia. Ann Clin Microbiol Antimicrob 2013; 12:11. doi: 10.1186/1476-0711-12-11 [Crossref] [ Google Scholar]
- Ahmed I, Rabbi MB, Sultana S. Antibiotic resistance in Bangladesh: a systematic review. Int J Infect Dis 2019; 80:54-61. doi: 10.1016/j.ijid.2018.12.017 [Crossref] [ Google Scholar]
- Ahmed AA, Hossain S, Aktar B, Juyee NA, Hasan SA. Prevalence of methicillin resistant Staphylococcus aureus in Khwaja Yunus Ali Medical College Hospital. KYAMC J 2016; 7(1):673-7. doi: 10.3329/kyamcj.v7i1.33756 [Crossref] [ Google Scholar]
- Roberts MC, Sutcliffe J, Courvalin P, Jensen LB, Rood J, Seppala H. Nomenclature for macrolide and macrolide-lincosamide-streptogramin B resistance determinants. Antimicrob Agents Chemother 1999; 43(12):2823-30. doi: 10.1128/aac.43.12.2823 [Crossref] [ Google Scholar]
- Islam MA, Alam MM, Choudhury ME, Kobayashi N, Ahmed MU. Determination of minimum inhibitory concentration (MIC) of cloxacillin for selected isolates of methicillin-resistant Staphylococcus aureus (MRSA) with their antibiogram. Bangladesh J Vet Med 2008; 6(1):121-6. [ Google Scholar]
- Mohammed SM. Use of cefoxitin as indicator for detection of methicillin resistant staphylococcus aureus. Baghdad Sci J 2011; 8(4):947-55. [ Google Scholar]
- Didier JP, Villet R, Huggler E, Lew DP, Hooper DC, Kelley WL. Impact of ciprofloxacin exposure on Staphylococcus aureus genomic alterations linked with emergence of rifampin resistance. Antimicrob Agents Chemother 2011; 55(5):1946-52. doi: 10.1128/aac.01407-10 [Crossref] [ Google Scholar]
- Siddiqui T, Muhammad IN, Khan MN, Naz S, Bashir L, Sarosh N. MRSA: prevalence and susceptibility pattern in health care setups of Karachi. Pak J Pharm Sci 2017; 30(6 Suppl):2417-21. [ Google Scholar]