Avicenna Journal of Clinical Microbiology and Infection. 9(2):81-87.
doi: 10.34172/ajcmi.2022.13
Original Article
Elevated Levels of Anti-SARS-Cov2 IgG Antibody in Health Care Workers in Hospitals From Hamadan Province, Iran: A Prospective Study
Ebrahim Jalili 1  , Saeid Bashirian 2, Mohammad Reza Faryabi 3, Mina Noroozbeygi 3, Ebrahim Daneshyar 1, Samereh Ghelichkhani 4, Salman Khazaei 5, Ghasem Solgi 3, *
, Saeid Bashirian 2, Mohammad Reza Faryabi 3, Mina Noroozbeygi 3, Ebrahim Daneshyar 1, Samereh Ghelichkhani 4, Salman Khazaei 5, Ghasem Solgi 3, * 
Author information:
1Department of Emergency Medicine, School of Medicine, Besat Hospital, Hamadan University of Medical Sciences, Hamadan, Iran
2Department of Public Health, Social Determinants of Health Research Center, Hamadan University of Medical Sciences, Hamadan, Iran
3Department of Immunology, School of Medicine, Besat Hospital, Hamadan University of Medical Sciences, Hamadan, Iran
4Department of Midwifery, School of Nursing and Midwifery, Hamadan University of Medical Sciences, Hamadan, Iran
5Department of Epidemiology, Research Center for Health Sciences, Hamadan University of Medical Sciences, Hamadan, Iran
*
Corresponding author: Ghasem Solgi, Department of Immunology, School of Medicine, Besat Hospital, Hamadan University of Medical Sciences, Hamadan, Iran Tel: +98 811 8380462, Fax: +98 811 8380208, P.O. Box: 6517838736. Email:
gh.solgi@umsha.ac.ir
Abstract
Aim: Seroprevalence among health care workers (HCWs) has been estimated in different studies in various regions and countries. This study aimed to screen the immunoglobulin M (IgM) and IgG seroprevalences and to assess the durability of IgG seropositivity, as well as the incidence of subsequent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in a group of Iranian HCWs.
Methods: This voluntary serological screening was prospectively performed on 800 HCWs (492 females and 308 males) in Hamadan between November 2020 and February 2021. Anti-SARS-CoV-2 IgG and IgM antibodies were assessed by the enzyme-linked immunosorbent assay method at two-time intervals.
Results: Overall, 243 out of 800 (30.38%) and 66 (8.25%) cases were IgG and IgM seropositive at their first antibody assessment, respectively. The male staff had a higher seroprevalence than females (31.49% vs. 29.67% for IgG, P=0.59 and 10.39% vs. 6.91% for IgM, P=0.08). Higher prevalences for both antibodies were found in the age group of 30-39.9 years (P=0.12 and P=0.05, respectively). In the second antibody screening, 81 (56.6%) cases were IgG seropositive. The mean titer of the first IgG antibody assessment in seropositive cases was lower than that of the second titer (2.95±2.07 vs. 5.08±4.01 cut-off index (COI), P=1.4×10-5 ). Moreover, the comparison of the first and second IgG titers among 81 seropositive cases demonstrated a significantly increased level of anti-SARS-CoV-2 antibody (5.08±4.01 vs. 3.49±2.41 COI, P=0.002).
Conclusions: Our findings revealed that the mean level of the anti-SARS-CoV-2 IgG antibody was significantly increased in the seropositive individuals after 2 months of follow-up.
Keywords: Seroprevalence, Antibody, IgG, SARS-CoV-2
Copyright and License Information
© 2022 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Jalili E, Bashirian S, Faryabi MR, Noroozbeygi M, Daneshyar E, Ghelichkhani S, et al. Elevated levels of Anti-SARS-Cov2 IgG antibody in health care workers in hospitals from hamadan province, iran: A prospective study. Avicenna J Clin Microbiol Infect. 2022; 9(2):81-87. doi:10.34172/ajcmi.2022.13
Introduction
The coronavirus disease 19 (COVID-19) infection caused by novel severe acute respiratory syndrome coronavirus 2(SARS-CoV-2) started a pandemic in 2020, and the World Health Organization reported it as a public health emergency issue concern. Overall, more than 226 million confirmed cases have been diagnosed worldwide, and more than 5 million cases have been reported in his regard in Iran until September 2021, while the status is updated daily (1). However, the estimates of the disease status are based on confirmed cases in symptomatic patients, and those for whom testing is unavailable are excluded from these cases. The symptoms included fever, chill, muscle pain, sore throat, cough, chest pain, and dyspnea, and in severe forms, the patient needed hospitalization (2). SARS-CoV-2 can be transmitted from individuals with asymptomatic and presymptomatic forms of the disease (3). Hence, it can increase the odds of infection in health care workers (HCWs) in the hospital due to higher contact with undiagnosed infections and confirmed patients. Therefore, health care professionals working in the hospital are at higher risk of SARS-CoV-2 infection.
The immune response to infection may be conferred by humoral and/or cellular immunity. Studies have demonstrated that the SARS-CoV Rp3 NP is a common protein among coronaviruses and is useful for diagnostic purposes (4). The development of the immunoglobulin M (IgM) antibody against SARS-CoV Rp3 NP protein during the early phase of COVID-19 infection is useful for the diagnosis of acute infection. However, IgG production takes a longer time to be produced and could be evidence of post-infection immunity even in asymptomatic cases (5). The investigation of post-infection immunity is identified by the functional correlates of protection and defined by endpoints such as the prevention of disease, hospitalization, and death (6). Post-infection immunity against SARS-CoV-2 infection occurred in most people, and reinfection with SARS-CoV-2 was rarely reported and mostly happened in subjects who experienced mild or asymptomatic primary infections (7,8). Moreover, some studies indicated that neutralizing antibodies could elicit post-infection immunity against SARS-CoV-2 infection (9-12).
Seroprevalence among HCWs has been estimated in a wide range of studies in different regions and countries. In Italy, the seroprevalence of HCWs was analyzed in May 2020 with 12% of seropositivity (13). In addition, the seroprevalence in hospital staff in the United Kingdom was estimated at more than 9% in April 2020 (14). Additionally, the seropositivity rate in the HCWs of different hospitals in Turkey was reported up to 6% among different job clusters between May and June 2020 (15). Nevertheless, a report from Ahmedabad, India, showed 23% seroprevalence among health care professionals in August 2020 (16). There was also a report from Gilan province in Iran, which determined the seroprevalence of 22% among household people in April 2020 (17).
In the current study, we performed a prospective evaluation of HCWs working in the hospital, including personnel who provide direct and indirect patient care, as well as non-clinical staff, to screen the IgM and IgG seroprevalences and to assess the stability of IgG seropositivity within 2 months of follow-up. Further, we examined the incidence of subsequent SARS-CoV-2 infection either as symptomatic infection or based on positive polymerase chain reaction (PCR) tests among seropositive cases during a short follow-up period.
Methods
After the approval of the Institutional Research Ethics Committee of Hamadan University of Medical Sciences (IR.UMSHA.REC.1399.806), this cross-sectional and prospective study evaluated the seroprevalence against SARS-CoV-2 in a group of volunteer individuals from different categories of health care professionals in 8 university hospitals in Hamadan province, Iran.
This voluntary serological screening was performed on 800 health care employees in the hospital, including 472 clinical and 328 non-clinical staff members between November 2020 and February 2021 (the first wave of the disease during the epidemic). All participants, including physicians (n = 23), nurses (n = 312), midwives (n = 30), laboratory personnel (n = 107), health care service staff (n = 70), and administrative staff (n = 258) provided written informed consent based on the Helsinki Declaration to participate in this prospective serosurvey study. All participants fulfilling the inclusion criteria such as working in the hospital that admits COVID-19 patients and having informed consent for participation in this investigation were randomly recruited into the study. Hospital staff with underlying diseases such as cardiovascular diseases, diabetes, autoimmune diseases, cancer, and other infectious diseases were excluded from the study.
The serological screening was performed at the time of recruitment and 2 months later (based on the availability of sera samples) for those seropositive individuals on the first measurement.
Serological Testing and Data Collection
IgG and IgM antibodies were detected against SARS-CoV-2 antigens (S1 and N proteins) by commercial enzyme-linked immunosorbent assay (ELISA) kits (SARS-CoV-2 IgG and SARS-CoV-2 IgM capture, Pishtazteb, Tehran, Iran) as per manufactures’ instructions. Furthermore, the second evaluation in seropositive subjects after 2 months was conducted using the IgG ELISA kit from the same company.
Demographic characteristics and probable SARS-CoV-2-related symptoms were recorded through a questionnaire-based approach at the time of sampling. The data included age, gender, job title, blood group, and some clinical symptoms such as fever, chill, headache, cough, lethargy, and myalgia that might be related to the SARS-CoV-2 infection.
Moreover, suspected cases (symptomatic or seropositive) in both steps of antibody screening were tested for the presence of SARS-CoV-2 RNA in nasopharyngeal swaps by the COVID-19 One-Step real-time (RT)-PCR kit based on the manufacturer’s instructions (Pishtazteb, Tehran, Iran).
Statistical Analysis
Data were analyzed with Stata 11 (Stata Corp., College Station, TX, USA). Frequency and proportion, as well as mean and standard deviation, were used for reporting categorical and continuous variables, respectively. Associations between categorical variables and IgG results were assessed for the second time using the chi-square test. The predictors of the presence of IgG and IgM antibodies in COVID-19 in HCWs were determined with a binary logistic regression model, and variables that were significantly associated with the IgG and IgM result in the crude model were included in the adjusted model. A P > 0.05 was considered a significant level.
Results
A total of 800 hospital employees, including 492 females (61.3%) and 308 males, with a mean age of 37.90 ± 8.6 years (age range: 20-65) were recruited for this prospective study. The hospital staff were categorized into different professions, including physicians (2.88%), nurses (39%), midwives (3.75%), laboratory personnel (13.38%), support services (8.75), and administrator staff (32.35%) and underwent the measurement of anti-SARS-CoV-2 IgM and IgG antibodies; based on the findings, 243 (30.38%) and 66 (8.25%) were IgG and IgM seropositive at their first antibody assessment, respectively. The self-reported symptoms among the study subjects were fever, lethargy, cough, loss of taste, nausea, and arthralgia. Among 243 seropositive staff, 116 cases were examined for the presence of SARS-CoV-2 RNA by the RT-PCR method, and only one subject was positive. Further, among 53 suspected cases with related symptoms who underwent CT scans, only 11 cases showed pulmonary involvement.
Overall, the male staff had a higher seroprevalence compared to females (31.49% vs. 29.67% for IgG and 10.39% vs. 6.91% for IgM antibody; P = 0.59 and P = 0.08, respectively, Table 1). Moreover, the highest seropositive prevalence for both antibodies was found in the age group of 30-39.9 years, although it was not statistically significant for IgG seropositive (34.89%, 95% CI: 0.92-2.14%, P = 0.12) and was marginally significant for IgM seropositive (9.97%, 95% CI: 0.99-5.32%, P = 0.05). Among 800 participants, 37.38% had an O blood group, which demonstrated a higher rate of seropositive for both IgG and IgM (33.78% and 10.03%, respectively) in comparison to other blood groups, but it was not statistically significant (P = 0.73). In addition, physicians and nurses represented a higher prevalence for both IgG (34.78% and 33.65%, respectively) and IgM (4.35% and 8.65%, respectively) seropositive compared to those in the administrative staff (27.13% IgG seropositive and 7.75% IgM seropositive, Table 1).
Table 1.
Distributions of IgG and IgM Seropositives Based on Different Risk Factors for COVID-19 Among Health Care Professionals (n = 800)
|
Variable
|
Total (%)
|
IgG Result
|
IgM Result
|
Negative
557 (69.63)
|
Positive
243 (30.38)
|
OR (95% CI)
|
P
Value
|
Negative
734 (91.75)
|
Positive
66 (8.25)
|
OR (95% CI)
|
P
Value
|
| Gender |
Female |
492 (61.50) |
346 (70.33) |
146 (29.67) |
1 |
- |
458 (93.09) |
34 (6.91) |
1 |
- |
| Male |
308 (38.50) |
211 (68.51) |
97 (31.49) |
1.09 (0.8, 1.48) |
0.59 |
276 (89.61) |
32 (10.39) |
1.56 (0.94, 2.59) |
0.08 |
| Age group (year) |
20-29.9 |
152 (19.00) |
110 (72.37) |
42 (27.63) |
1 |
- |
145 (95.39) |
7 (4.61) |
1 |
- |
| 30-39.9 |
321 (40.13) |
209 (65.11) |
112 (34.89) |
1.4 (0.92, 2.14) |
0.12 |
289 (90.03) |
32 (9.97) |
2.29 (0.99, 5.32) |
0.05 |
| 40-49.9 |
231 (28.88) |
169 (73.16) |
62 (26.84) |
0.96 (0.61, 1.52) |
0.86 |
211 (91.34) |
20 (8.66) |
1.96 (0.81, 4.76) |
0.14 |
| 50-60 |
96 (12.00) |
69 (71.88) |
27 (28.13) |
1.02 (0.58, 1.8) |
0.93 |
89 (92.71) |
7 (7.29) |
1.63 (0.55, 4.8) |
0.38 |
| Blood group |
A |
230 (28.75) |
167 (72.61) |
63 (27.39) |
1 |
- |
209 (90.87) |
21 (9.13) |
1 |
- |
| B |
180 (22.50) |
124 (68.89) |
56 (31.11) |
1.2 (0.78, 1.84) |
0.41 |
171 (95.00) |
9 (5.00) |
0.52 (0.23, 1.17) |
0.12 |
| AB |
91 (11.38) |
68 (74.73) |
23 (25.27) |
0.9 (0.51, 1.56) |
0.7 |
85 (93.41) |
6 (6.59) |
0.7 (0.27, 1.8) |
0.46 |
| O |
299 (37.38) |
198 (66.22) |
101 (33.78) |
1.35 (0.93, 1.97) |
0.12 |
269 (89.97) |
30 (10.03) |
1.11 (0.62, 1.99) |
0.73 |
Job
categories |
Physician |
23 (2.88) |
15 (65.22) |
8 (34.78) |
1.43(0.58, 3.53) |
0.43 |
22 (95.65) |
1 (4.35) |
0.54 (0.07, 4.22) |
0.56 |
| Nurse |
312 (39) |
207 (66.35) |
105 (33.65) |
1.36 (0.95, 1.95) |
0.09 |
285 (91.35) |
27 (8.65) |
1.13 (0.62, 2.06) |
0.7 |
| Midwife |
30 (3.75) |
22 (73.33) |
8 (26.67) |
0.98 (0.42, 2.3) |
0.96 |
30 (100.00) |
0 |
- |
- |
| Service |
70 (8.75) |
50 (71.43) |
20 (28.57) |
1.07 (0.6, 1.93) |
0.81 |
65 (92.86) |
5 (7.14) |
0.92 (0.33, 2.53) |
0.87 |
| Laboratory |
107 (13.38) |
75 (70.09) |
32 (29.91) |
1.15 (0.7, 1.88) |
0.59 |
94 (87.85) |
13 (12.15) |
1.65 (0.79, 3.44) |
0.19 |
| Officials |
258 (32.25) |
188 (72.87) |
70 (27.13) |
1 |
- |
238 (92.25) |
20 (7.75) |
1 |
- |
Note. IgG: Immunoglobulin G; IgM: Immunoglobulin M; COVID: Coronavirus disease; OR: Odds ratio; CI: Confidence interval.
Furthermore, the participants who experienced fever and cough showed higher odds for seropositivity (OR > 1), while those who experienced shortness of breath, sweating, headache, abdominal pain, and myalgia demonstrated the least odds for seropositivity (OR > 0.0, Figure 1).
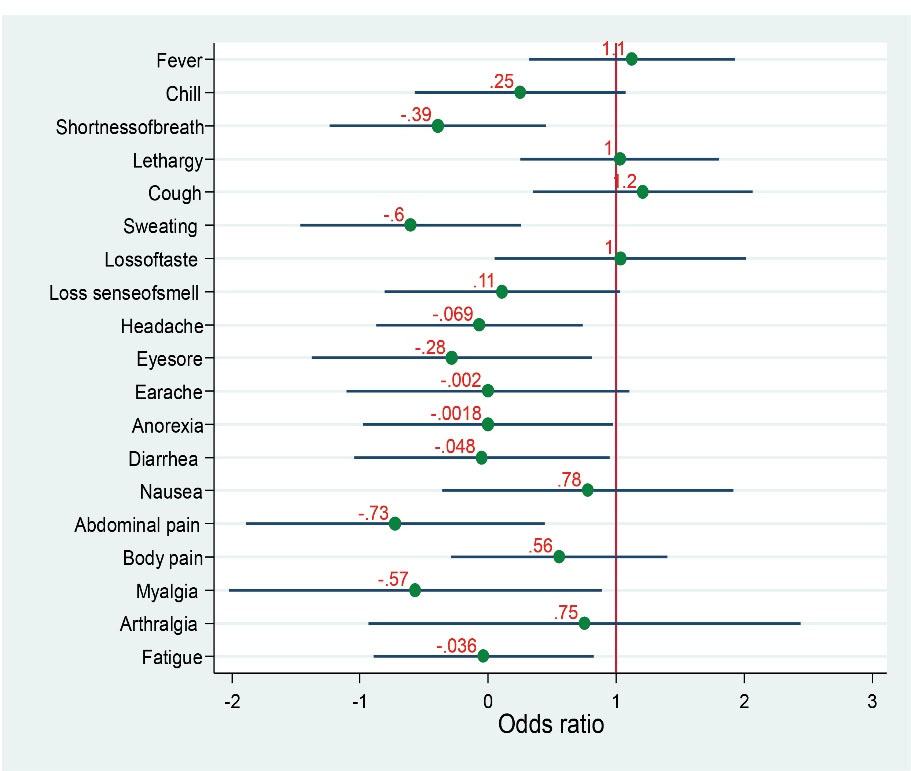
Figure 1.
Estimated Odds Ratios of Seroprevalence for IgG Antibody Based on the Presence of Different Possibly SARS-CoV-2 Related Symptoms. Note. IgG, Immunoglobulin G; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2.
.
Estimated Odds Ratios of Seroprevalence for IgG Antibody Based on the Presence of Different Possibly SARS-CoV-2 Related Symptoms. Note. IgG, Immunoglobulin G; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2.
Second Anti-SARS-CoV-2 IgG Assessment Among Seropositive Subjects
Two months after the first antibody screening, 143 out of 243 IgG seropositive participants were available to be re-tested for IgG assessment and preceded by PCR testing for SARS-CoV-2 infection. Of these, 81 participants (56.6%) were still IgG seropositive. Additionally, 18 cases had laboratory-confirmed SARS-CoV-2 infection (by RT-PCR) and 22 had been affected with different forms of COVID-19 during the last 2 months (including 14 mild, 6 moderate, and 2 severe forms of the disease); surprisingly, 12 of 22 cases were IgG seronegative. The most common symptoms among IgG seropositive were fever (44.44%), cough (40.74%), lethargy (40.52%), chill (37.04%), and anorexia (29.63%). Interestingly, the frequencies of fatigue, headache, and nausea symptoms were higher in the IgG seronegative compared to seropositive (Figure 2).
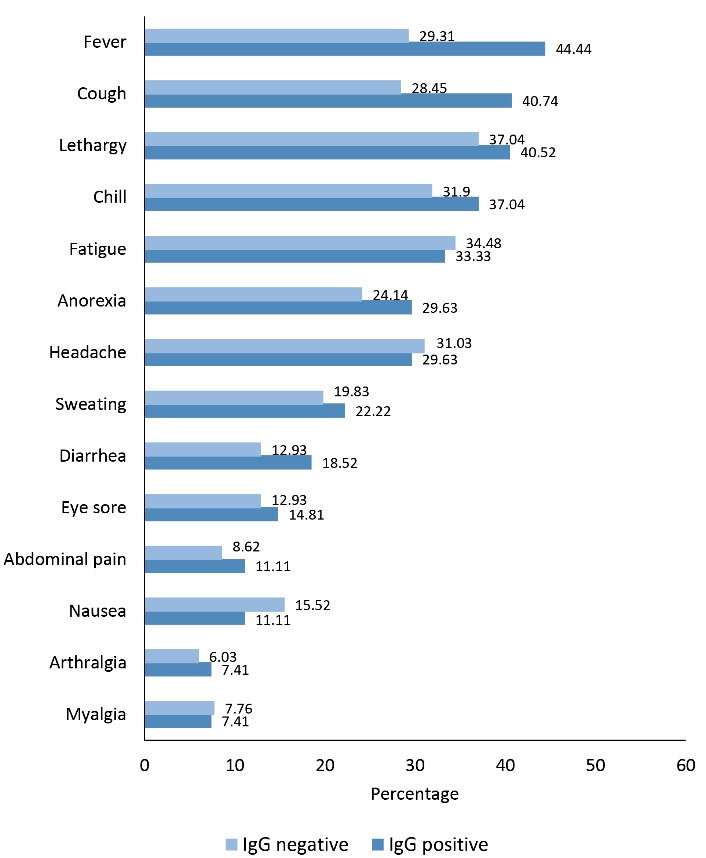
Figure 2.
Comparison of the Frequencies of Possibly Related SARS-CoV-2 Symptoms Between IgG Seropositive and Seronegative at the Second Antibody Screening Test. Note. IgG, Immunoglobulin G; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2.
.
Comparison of the Frequencies of Possibly Related SARS-CoV-2 Symptoms Between IgG Seropositive and Seronegative at the Second Antibody Screening Test. Note. IgG, Immunoglobulin G; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2.
Moreover, the prevalence of the second IgG seropositive was higher in the female group, the age group of 30-39.9 years, O blood group, and nurses, but there were no statistically significant differences in comparison to other groups of variables (Table 2).
Table 2.
Second IgG Assessment in 143 Seropositive in Terms of Demographics Features
|
Variables
|
Total (%)
|
Negative
|
Positive
|
P
Value
|
| Gender |
Female |
80 (55.94) |
36 (45.00) |
44 (55.00) |
0.66 |
| Male |
63 (44.06) |
26 (41.27) |
37 (58.73) |
| Age group (y) |
20-29.9 |
18 (12.59) |
6 (33.33) |
12 (66.67) |
0.55 |
| 30-39.9 |
60 (41.96) |
30 (50.00) |
30 (50.00) |
| 40-49.9 |
45 (31.47) |
18 (40.00) |
27 (60.00) |
| 50-60 |
20 (13.99) |
8 (40.00) |
12 (60.00) |
| Blood group |
A |
42 (29.37) |
20 (47.62) |
22 (52.38) |
0.23 |
| B |
30 (20.98) |
16 (53.33) |
14 (46.67) |
| AB |
16 (11.19) |
8 (50.00) |
8 (50.00) |
| O |
55 (38.46) |
18 (32.73) |
37 (67.27) |
| Job categories |
Physician |
8 (5.59) |
4 (50.00) |
4 (50.00) |
0.49 |
| Nurse |
58 (40.56) |
29 (50.00) |
29 (50.00) |
| Midwife |
3 (2.10) |
0 |
3 (100.00) |
| Service |
12 (8.39) |
4 (33.33) |
8 (66.67) |
| Laboratory |
17 (11.89) |
6 (35.29) |
11 (64.71) |
| Officials |
45 (31.47) |
19 (42.22) |
26 (57.78) |
Note. IgG: Immunoglobulin G.
The mean IgG antibody titer in 243 participants, who were IgG seropositive at the first assay, was lower than that of 81 IgG seropositive at the second antibody screening (2.95 ± 2.07 and 5.08 ± 4.01 cut-off index (COI), respectively, P = 1.4×10-5). Further, a comparison of the first and the second IgG titers among those 81 seropositive revealed a significantly increased level of antibody (5.08 ± 4.01 vs. 3.49 ± 2.41 COI, P = 0.002). Furthermore, 39 out of 800 participants had both IgM and IgG seropositive, and after two months, 18 of them were still IgG seropositive and 2 of them experienced a moderate form of infection.
Discussion
The monumental pressure on the health care system during the pandemic situation has made the protection of first-line HCWs against infection an important concern. As reported in Spain, the nationwide seroprevalence was found at 5%, while it has reached more than 8% in HCWs (19). Moreover, it has been determined that antibody production against SARS-CoV-2 could induce protection against reinfection (10). In the current study, we determined the prevalence of IgM and IgG seropositive among different hospital staff and recalled some of the available IgG seropositive after two months to assess the stability of IgG seropositive results.
The IgM and IgG seroprevalences were found at 8.25% and 30.38%, respectively. However, no significant differences were observed based on gender, age, blood group, and different job categories. In addition, 4.87% of cases were both IgM and IgG seropositive. Studies on determining IgG seroprevalence in health care professionals reported different results ranging from 4% in Denmark to 23% in India (14-16,20). The most important issue for the interpretation and comparison of the results was the time of sampling, as the above-mentioned studies had been conducted within a few months after the initiation of the pandemic, while our study was performed about one year later, thus a higher incidence of infection was observed among the population. Furthermore, many European and American countries had enforced strong lockdown policies to control the disease spread, which could somewhat explain the differences between our results and those of other studies. Of note, a previous report from Gilan province in our country estimated 22% IgG seroprevalence among the household population (17), which is in line with our results among HCWs.
Likewise, the second antibody assessment after two months revealed that 81 out of 143 (56.6%) seropositive cases had still high serum levels of IgG with a considerable increase compared to the first measurement. Although it has been demonstrated that antibodies against SARS-CoV-2 antigens could persist for up to 8 months (21,22), we observed that less than half of seropositive participants converted to the seronegative status after 2 months, which is in conformity with the results of other studies on HCWs (23,24). However, it has been reported that the speed of antibody decrease in the patients depends on disease severity, and those with mild symptoms had a rapid reduction of antibody levels (25). We also found that those who had a fever, cough, loss of tasting sense, and lethargy symptoms at the time of sample collection, had more odds of being IgG seropositive.
In addition, among 143 subjects for the second IgG antibody screening, 22 cases were found with the symptomatic disease during 2 months of follow-up. Based on the results of antibody screening tests, 10 of those 22 participants were IgG seronegative and experienced a mild or moderate form of the disease. There could raise the question of whether they are susceptible to reinfection. It was indicated that 10% of PCR-positive COVID-19 patients showed seronegative results after mild disease while they had reactive specific T cells (26), indicating the importance of cellular immunity against infection. Therefore, more studies are warranted on different aspects of immune protection in such cases. Additionally, it was revealed that the level of antiviral antibodies is not correlated with disease mortality and severity, while the antibody production a few days before the disease onset was considered a recovery factor (27). Hence, further studies are required to clarify the emerging and disappearing and kinetics of antibodies in terms of the sampling time, results of the PCR test, and time of the disease onset.
Notably, 2 subjects in our study experienced a severe form of COVID-19, even though they were seropositive both at the first and second antibody assessments. Reinfection reports are rare, and it has been reported that no increasing trend was observed up to seven months following the first antibody-positive test (28). Nevertheless, repeated exposure and a highly infected environment may increase the risk of reinfection in HCWs. Accordingly, we observed that the high burden of symptomatic and asymptomatic patients in the hospitals and high contact of first-line HCWs have led to an increasing IgG titer in seropositive participants up to 2 folds after two months, highlighting the necessity of providing suitable personal protective equipment and considering more options for the protection of HCWs (29,30). Our results must be interpreted with caution due to the unavailability of the sera samples from all seropositive participants for the second antibody assessment and consequently reduced sample size.
In conclusion, our results revealed high COVID-19 IgG seroprevalence among HCWs in Hamadan province, Iran after one year since the pandemic initiation. The seropositivity was stable after two months in more than half of the seropositive. Moreover, nearly 7% of seronegative cases in the second IgG assay had experienced mild to moderate disease. No significant differences were found regarding the blood group, age, and professions stratification. The increasing antibody level was observed in HCWs, remarkably nurses, as a result of high contact with COVID-19 patients. The infection with SARS-CoV-2 was observed even in the presence of a high antibody titer that could be indicative of insufficiency of protection made by antibodies. These findings confirmed that natural infection could not induce acceptable protection against the disease, which might indicate the necessity of the vaccination of HCWs to elicit higher protective antibody production. The high number of seropositive HCWs highlights the need for regular protective measures to protect both staff and patients from possible nosocomial transmission. Further longitudinal serological studies and evaluation of other aspects of immunologic responses could help clarify the quality and persistence of SARS-CoV-2-specific immunity among HCWs.
Acknowledgments
This serosurvey study was financially supported by the Vice-chancellor for Research and Technology, Hamadan University of Medical Sciences (Grant No: 9910167154), Hamadan, Iran.
Conflict of Interests
The authors declare that they have no conflict of interests.
Ethical Approval
Institutional Research Ethics Committee of Hamadan University of Medical Sciences approved the study (IR.UMSHA.REC.1399.806).
References
- Coronavirus Disease (COVID-19) Pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
- Yuki K, Fujiogi M, Koutsogiannaki S. COVID-19 pathophysiology: a review. Clin Immunol 2020; 215:108427. doi: 10.1016/j.clim.2020.108427 [Crossref] [ Google Scholar]
- Hu S, Wang W, Wang Y, Litvinova M, Luo K, Ren L. Infectivity, susceptibility, and risk factors associated with SARS-CoV-2 transmission under intensive contact tracing in Hunan, China. Nat Commun 2021; 12(1):1533. doi: 10.1038/s41467-021-21710-6 [Crossref] [ Google Scholar]
- Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020; 579(7798):270-3. doi: 10.1038/s41586-020-2012-7 [Crossref] [ Google Scholar]
- Zhang W, Du RH, Li B, Zheng XS, Yang XL, Hu B. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect 2020; 9(1):386-9. doi: 10.1080/22221751.2020.1729071 [Crossref] [ Google Scholar]
- Plotkin SA. Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis 2008; 47(3):401-9. doi: 10.1086/589862 [Crossref] [ Google Scholar]
- Van Elslande J, Vermeersch P, Vandervoort K, Wawina-Bokalanga T, Vanmechelen B, Wollants E. Symptomatic Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Reinfection by a Phylogenetically Distinct Strain. Clin Infect Dis 2021; 73(2):354-6. doi: 10.1093/cid/ciaa1330 [Crossref] [ Google Scholar]
- Gupta V, Bhoyar RC, Jain A, Srivastava S, Upadhayay R, Imran M. Asymptomatic reinfection in 2 healthcare workers from India with genetically distinct severe acute respiratory syndrome coronavirus 2. Clin Infect Dis 2021; 73(9):e2823-e5. doi: 10.1093/cid/ciaa1451 [Crossref] [ Google Scholar]
- Addetia A, Crawford KHD, Dingens A, Zhu H, Roychoudhury P, Huang ML. Neutralizing antibodies correlate with protection from SARS-CoV-2 in humans during a fishery vessel outbreak with a high attack rate. J Clin Microbiol 2020; 58(11):e02107-20. doi: 10.1128/jcm.02107-20 [Crossref] [ Google Scholar]
- Mumoli N, Vitale J, Mazzone A. Clinical immunity in discharged medical patients with COVID-19. Int J Infect Dis 2020; 99:229-30. doi: 10.1016/j.ijid.2020.07.065 [Crossref] [ Google Scholar]
- Ripperger TJ, Uhrlaub JL, Watanabe M, Wong R, Castaneda Y, Pizzato HA, et al. Orthogonal SARS-CoV-2 serological assays enable surveillance of low-prevalence communities and reveal durable humoral immunity. Immunity 2020;53(5):925-33.e4. 10.1016/j.immuni.2020.10.004.
- Payne DC, Smith-Jeffcoat SE, Nowak G, Chukwuma U, Geibe JR, Hawkins RJ. SARS-CoV-2 infections and serologic responses from a sample of U.S. Navy service members - USS Theodore Roosevelt, April 2020. MMWR Morb Mortal Wkly Rep 2020; 69(23):714-21. doi: 10.15585/mmwr.mm6923e4 [Crossref] [ Google Scholar]
- Poletti P, Tirani M, Cereda D, Guzzetta G, Trentini F, Marziano V. Seroprevalence of and risk factors associated with SARS-CoV-2 infection in health care workers during the early COVID-19 pandemic in Italy. JAMA Netw Open 2021; 4(7):e2115699. doi: 10.1001/jamanetworkopen.2021.15699 [Crossref] [ Google Scholar]
- Lumley SF, O’Donnell D, Stoesser NE, Matthews PC, Howarth A, Hatch SB. Antibody status and incidence of SARS-CoV-2 infection in health care workers. N Engl J Med 2021; 384(6):533-40. doi: 10.1056/NEJMoa2034545 [Crossref] [ Google Scholar]
- Alkurt G, Murt A, Aydin Z, Tatli O, Agaoglu NB, Irvem A. Seroprevalence of coronavirus disease 2019 (COVID-19) among health care workers from three pandemic hospitals of Turkey. PLoS One 2021; 16(3):e0247865. doi: 10.1371/journal.pone.0247865 [Crossref] [ Google Scholar]
- Prakash O, Solanki B, Sheth J, Makwana G, Kadam M, Vyas S. SARS-CoV2 IgG antibody: seroprevalence among health care workers. Clin Epidemiol Glob Health 2021; 11:100766. doi: 10.1016/j.cegh.2021.100766 [Crossref] [ Google Scholar]
- Shakiba M, Nazemipour M, Salari A, Mehrabian F, Nazari SSH, Rezvani SM. Seroprevalence of SARS-CoV-2 in Guilan province, Iran, April 2020. Emerg Infect Dis 2021; 27(2):636-8. doi: 10.3201/eid2702.201960 [Crossref] [ Google Scholar]
- Pollán M, Pérez-Gómez B, Pastor-Barriuso R, Oteo J, Hernán MA, Pérez-Olmeda M. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet 2020; 396(10250):535-44. doi: 10.1016/s0140-6736(20)31483-5 [Crossref] [ Google Scholar]
- Montenegro P, Brotons C, Serrano J, Fernández D, Garcia-Ramos C, Ichazo B. Community seroprevalence of COVID-19 in probable and possible cases at primary health care centres in Spain. Fam Pract 2021; 38(2):154-9. doi: 10.1093/fampra/cmaa096 [Crossref] [ Google Scholar]
- Galanis P, Vraka I, Fragkou D, Bilali A, Kaitelidou D. Seroprevalence of SARS-CoV-2 antibodies and associated factors in healthcare workers: a systematic review and meta-analysis. J Hosp Infect 2021; 108:120-34. doi: 10.1016/j.jhin.2020.11.008 [Crossref] [ Google Scholar]
- Choe PG, Kim KH, Kang CK, Suh HJ, Kang E, Lee SY. Antibody responses 8 months after asymptomatic or mild SARS-CoV-2 infection. Emerg Infect Dis 2021; 27(3):928-31. doi: 10.3201/eid2703.204543 [Crossref] [ Google Scholar]
- Hartley GE, Edwards ESJ, Aui PM, Varese N, Stojanovic S, McMahon J. Rapid generation of durable B cell memory to SARS-CoV-2 spike and nucleocapsid proteins in COVID-19 and convalescence. Sci Immunol 2020; 5(54):eabf8891. doi: 10.1126/sciimmunol.abf8891 [Crossref] [ Google Scholar]
- Kiefer MK, Allen KD, Russo JR, Ma’ayeh M, Gee SE, Kniss D. Decline in Sars-CoV-2 antibodies over 6-month follow-up in obstetrical healthcare workers. Am J Reprod Immunol 2021; 86(6):e13490. doi: 10.1111/aji.13490 [Crossref] [ Google Scholar]
- Patel MM, Thornburg NJ, Stubblefield WB, Talbot HK, Coughlin MM, Feldstein LR. Change in antibodies to SARS-CoV-2 over 60 days among health care personnel in Nashville, Tennessee. JAMA 2020; 324(17):1781-2. doi: 10.1001/jama.2020.18796 [Crossref] [ Google Scholar]
- Ibarrondo FJ, Fulcher JA, Goodman-Meza D, Elliott J, Hofmann C, Hausner MA. Rapid decay of anti-SARS-CoV-2 antibodies in persons with mild COVID-19. N Engl J Med 2020; 383(11):1085-7. doi: 10.1056/NEJMc2025179 [Crossref] [ Google Scholar]
- Steiner S, Schwarz T, Corman VM, Sotzny F, Bauer S, Drosten C. Reactive T cells in convalescent COVID-19 patients with negative SARS-CoV-2 antibody serology. Front Immunol 2021; 12:687449. doi: 10.3389/fimmu.2021.687449 [Crossref] [ Google Scholar]
- Lucas C, Klein J, Sundaram ME, Liu F, Wong P, Silva J. Delayed production of neutralizing antibodies correlates with fatal COVID-19. Nat Med 2021; 27(7):1178-86. doi: 10.1038/s41591-021-01355-0 [Crossref] [ Google Scholar]
- Abu-Raddad LJ, Chemaitelly H, Coyle P, Malek JA, Ahmed AA, Mohamoud YA. SARS-CoV-2 antibody-positivity protects against reinfection for at least seven months with 95% efficacy. EClinicalMedicine 2021; 35:100861. doi: 10.1016/j.eclinm.2021.100861 [Crossref] [ Google Scholar]
- Murongazvombo AS, Jones RS, Rayment M, Mughal N, Azadian B, Donaldson H. Association between SARS-CoV-2 exposure and antibody status among healthcare workers in two London hospitals: a cross-sectional study. Infect Prev Pract 2021; 3(3):100157. doi: 10.1016/j.infpip.2021.100157 [Crossref] [ Google Scholar]
- Milazzo L, Lai A, Pezzati L, Oreni L, Bergna A, Conti F. Dynamics of the seroprevalence of SARS-CoV-2 antibodies among healthcare workers at a COVID-19 referral hospital in Milan, Italy. Occup Environ Med 2021; 78(8):541-7. doi: 10.1136/oemed-2020-107060 [Crossref] [ Google Scholar]