Avicenna Journal of Clinical Microbiology and Infection. 9(1):41-48.
doi: 10.34172/ajcmi.2022.07
Review Article
Prevalent ABO Blood Groups in Alive and Dead COVID-19 Patients: A Systematic Review and Meta-analysis
Amir Emami 1, *  , Fatemeh Javanmardi 2, Ali Akbari 3, Neda Pirbonyeh 1
, Fatemeh Javanmardi 2, Ali Akbari 3, Neda Pirbonyeh 1
Author information:
1Department of Microbiology, Burn and Wound healing research center, Shiraz University of Medical Sciences, Shiraz, Iran
2Department of Biostatistics, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran
3Department of Anesthesiology, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran
Abstract
Background: The rapid spread of the virus around the world is raising alarms among scientists to identify vulnerable people who are at greater risk of infection. In this regard, the present study aimed to determine the prevalence of severe acute respiratory syndrome coronavirus 2 in different blood groups.
Methods: To find relevant studies, a comprehensive and systematic search was conducted based on the PRISMA guidelines in international databases such as PubMed, Scopus, Web of Sciences, and Google Scholar by December 31, 2020.
Results: After the audit and exclusion of double and unrelated studies, 19 articles were included in the analysis. The most prevalent blood types in alive patients were A and O which calculated the aggregate prevalence at - 39.06 (95% CI: 36.22-41.94) and 35.60 (95% CI: 32.48-38.79). In addition, patients with blood groups B and AB were less than two other groups. The aggregated/estimated prevalence was 7.72 (95% CI: 5.06-10.88) and 16.23 (95% CI: 12.86-19.91) for AB and B, respectively. The results for the deceased had a similar pattern that was high for blood types A and O.
Conclusions: The current meta-analysis validated different prevalence rates of blood group types in patients with COVID-19, confirming that types A and O blood groups are the most prevalent types of deaths and live patients.
Keywords: COVID-19, ABO blood group, Alive, Dead, Meta-analysis
Copyright and License Information
© 2022 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Emami A, Javanmardi F, Akbari A, Pirbonyeh N. Prevalent ABO blood groups in alive and dead COVID-19 patients: a systematic review and meta-analysis.Avicenna J Clin Microbiol Infect. 2022; 9(1):41-48. doi:10.34172/ajcmi.2022.07
Background
Since the beginning of the 2019 coronavirus pandemic, unprecedented extensive research and efforts have been provoked by several scientists and doctors to clarify and increase our understanding of characteristics, risk factors, clinical manifestations, treatment options, and management strategies related to this disease (1,2). The globally rapid spread of the virus is raising alarms among scientists to identify vulnerable individuals at higher risk of infection (3). An extensive variation has so far been observed in the pattern of the disease and the frequency of the occurrence of different symptoms among infected patients with severe acute respiratorysyndrome coronavirus 2 (SARS-CoV-2). It has been presented as mild, moderate, severe cases, and some isolated fatal consequences (4). The development of the disease in various forms is associated with many factors such as old age, male gender, preexisting cardiovascular diseases, hypertension, and diabetes (5,6). Based on the previous pandemic created by the Coronavirus family, namely, SARS and Middle East respiratory syndrome (MERS), it was found that the risk factors of these diseases depend on several types of chemical and physical bonds formed between the virus and the host cells (7).
In previous studies, the ABO blood group was reported to contribute to the risk of multiple infectious diseases. According to some reports, the presence of blood group O might significantly decrease the risk of viral hepatitis while the distribution of the virus is higher between Rh-positive donors. Meanwhile, the system can play a direct role in infection by serving as receptors or coreceptors for microorganisms, parasites, and viruses.
Human histo-blood group antigens (surface antigens) of human red blood cells represent polymorphic traits inherited among individuals and populations, and these differences in blood group antigens can increase or decrease the host susceptibility to many infections (8,9). Similar effects of blood group types were observed in other infectious diseases including Plasmodium falciparum, Helicobacter pylori, Norwalk virus, and Neisseria gonorrhea which are more severe in non-O blood groups (10). Most of the reports about COVID-19 and its association with blood groups have dealt with the severity of the disease. This study sought to understand the prevalence of SARS-CoV-2 in different blood groups.
Methods
Search Strategy
In the current study, Reporting Items for Systematic Review and Meta-analysis guidelines were used, and the initial protocol was registered in PROSPERO (CRD42020216106, https://www.crd.york.ac.uk/prospero/#searchadvanced).
To find relevant studies, a comprehensive and systematic search was conducted in several international databases including PubMed, Scopus, Web of Sciences, and Google Scholar until 31 October 2020. Some mesh keywords were applied for the search strategy, including (COVID-19 OR SARS-CoV-2) AND (“blood group” OR “ABO”), “COVID-19” OR “SARS-CoV-2” AND “blood group” OR “ABO”. More manual evaluations were conducted in the reference section of proper articles. Search terms were limited to the English language, but due to the nature of the disease which has started in China, major articles were in Chinese, thus their English abstracts were assessed for these studies.
Inclusion and Exclusion Criteria
The intended studies were independently evaluated by two authors. In case of disagreement, the third author decided about it. In this assessment, Newcastle Ottawa Scale was employed which consists of selection, comparability, and exposure parts (11). Scores varied between 0 and 9, and studies were excluded from the analysis if their scores were less than five. The results of the quality assessment are provided in Table S1 (Supplementary File 1). The inclusion criterion was reporting the total number of infected patients with SARS-CoV-2, the total number of death cases, and the number of ABO blood groups. Case reports, letters, and review articles were excluded from the analysis. Table 1 presents the characteristics of the included studies.
Table 1.
Characteristics of Included Studies in Analysis
|
Authors
|
Type of Study
|
Total Control
|
Total Case
|
Died
Cases
|
Blood Group Types
|
|
A
|
B
|
AB
|
O
|
|
Case
|
Control
|
Case
|
Control
|
Case
|
Control
|
Case
|
Control
|
| Abdollahiet al (12) |
Cross-Sectional |
500 |
397 |
N.M |
160 (40.3) |
180 (360 |
89 (22.4) |
105 (210 |
37 (9.3) |
25 (5)
|
111 (28) |
190 (38) |
| Ad’hiah et al (13) |
Case-Control |
595 |
300 |
|
86 (28.7) |
186 (31.3) |
80 (26.7) |
142 (23.9) |
59 (19.6) |
62 (10.4) |
75 (25) |
205 (34.4) |
|
|
|
|
37 |
20 (54.05) |
|
3 (8.10) |
|
12 (32.43) |
|
2 (5.40) |
|
| Sardu et al (14) |
|
|
164 |
|
N.M |
|
N.M |
|
N.M |
|
72 (43.90) |
|
| Prospective |
|
|
24 |
N.M |
|
N.M |
|
N.M |
|
6 (25) |
|
| Latz et al (15) |
Retrospective |
|
1289 |
|
440 (34.13) |
|
201 (15.59) |
|
61 (4.73) |
|
587 (45.53) |
|
|
|
|
|
89 |
36 (40.44) |
|
14 (15.73) |
|
5 (5.61) |
|
34 (38.2) |
|
| Göker et al (16) |
Case-Control |
1882 |
186 |
N.M |
106 (57) |
716 (38) |
20 (10.8) |
277 (14.7) |
14 (7.5) |
188 (10) |
46 (24.8) |
701 (37.2) |
| Ahmed et al (17) |
Short Letter |
269 |
86 |
N.M |
34 (40) |
92 (34) |
19 (22) |
48 (18) |
12 (14) |
17 (6) |
21 (24) |
112 (42) |
| Zhao et al (18) |
|
3694 |
1775 |
|
670 (37.8) |
1188 (32.2) |
469 (26.4) |
920 (24.9) |
178 (10) |
336 (9.1) |
458 (25.8) |
1250 (33.8) |
| Case-Control |
|
|
206 |
85 (41.26) |
|
50 (24.27) |
|
19 (9.22) |
|
52 (25.24) |
|
| Li et al (19) |
Case-Control |
3694 |
265 |
|
104 (39.3) |
1188 (32.2) |
67 (25.30 |
920 (24.9) |
26 (9.80) |
336 (9.1) |
68 (25.7) |
1250 (33.8) |
|
|
|
|
57 |
20 (35.08) |
|
15 (26.31) |
|
8 (14.03) |
|
14 (24.56) |
|
| Boudin et al (20) |
Case-Control |
409 |
1279 |
N.M |
521 (40.7) |
153 (37.4) |
135 (10.6) |
48 (11.7) |
54 (4.2) |
16 (3.9) |
553 (43.2) |
189 (46.2) |
| Valenti et al (20) |
Case-Control |
890 |
505 |
N.M |
225 (44.6) |
339 (38.1) |
53 (10.5) |
106 (11.9) |
37 (7.3) |
29 (3.3) |
190 (37.6) |
416 (46.7) |
| Zietz et al (21) |
|
|
2394 |
|
786 (32.83) |
|
392 (16.37) |
|
94 (3.92) |
|
1122 (46.86) |
|
| Cohort |
|
|
331 |
104 (31.4) |
|
46 (13.89) |
|
15 (4.53) |
|
166 (50.15) |
|
| Barnkob et al (22) |
|
|
7422 |
|
3296 |
199211 |
897 |
52838 |
378 |
20782 |
2851 |
193401 |
| Retrospective Cohort |
|
|
550 |
259 |
|
64 |
|
26 |
|
201 |
|
| Fan et al (23) |
Case-Control |
103 |
105 |
N.M |
45 (42.8) |
30 (29.1) |
28 (26.7) |
32 (31.1) |
9 (8.57) |
11 (10.7) |
23 (21.9) |
30 (29.1) |
| Leaf et al (24) |
Observational Study |
|
2033 |
|
666 (32.75) |
|
328 (16.33) |
|
89 (4.37) |
|
950 (46.72) |
|
|
|
|
|
799 |
268 (33.54) |
|
129 (16.14) |
|
41 (5.13) |
|
361 (45.18) |
|
| Marcos et al (25) |
Case Control |
182384 |
226 |
N.M |
99 (44) |
78402 (43) |
19 (8.4) |
11725 (6.4) |
10 (4.4) |
4652 (2.6) |
97 (43.1) |
87605 (48) |
| Taha et al (26) |
Case-Control |
1000 |
557 |
N.M |
180 (32.1) |
272 (27.2) |
102 (18.3) |
191 (19.1) |
34 (6.1) |
34 (4) |
241 (43.3) |
503 (50.3) |
| Ray et al (27) |
Cohort |
|
7071 |
|
2420 (3) |
|
390 (3.8) |
|
1378 (4.1) |
|
2883 (2.9) |
|
| Diaz et al (28) |
Case-Control |
75870 |
854 |
|
403 (47.19) |
31880 (42.02) |
65 (7.61) |
5705 (7.52) |
32 (3.75) |
2367 (3.12) |
354 (41.45) |
35918 (47.34) |
| Wu et al (29) |
Case-Control |
1991 |
187 |
N.M |
69 (36.9) |
547 (27.47) |
63 (33.69) |
644 (32.35) |
14 (7.49) |
199 (9.99) |
41 (21.92) |
601 (30.19) |
Statistical Analysis
To estimate the prevalence of infected and death cases in different blood group types, the inverse-variance weighted method was considered by applying the Stata commandmetaprop. Higgins I2 and Cochrane Q statistics for the heterogeneity evaluation, which was defined as low (I2 < 25%), high (I2 > 50%), and moderate (25-50%). In case of high heterogeneity, the random effect model was performed using the DerSimonian and Laird method. Graphical results were displayed through the forest plot. A statistical significance was considered to be less than 5%. All statistical analyses were performed by Stata (Version 13, StataCorp, 2019. Stata Statistical Software: Release 13. College Station, TX).
Results
Study Screening
The initial literature search yielded 150 studies. After removing duplications, 65 articles were screened based on their titles and abstracts. The full texts of 23 articles were reviewed, and their references were assessed for further publications. Based on this review, 6 articles were excluded due to non-compliance with the inclusion criteria. Finally, the current meta-analysis comprised a total of 19 studies in the qualitative synthesis. Figure 1 shows this systematic literature review process.
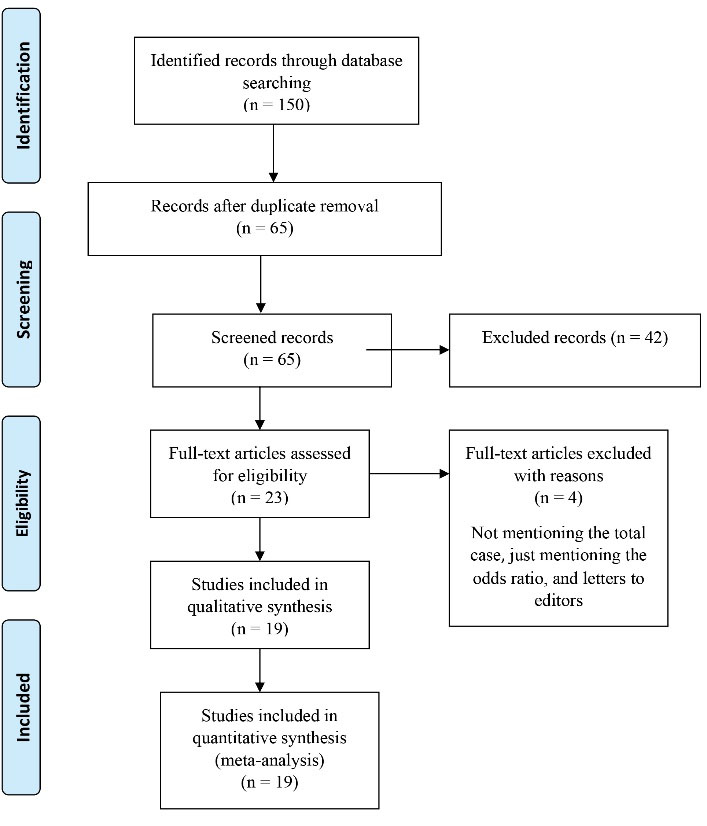
Figure 1.
PRISMA Chart Through Different Phases of the Systematic Review. Note. PRISMA: Reporting Items for Systematic Review and Meta-analysis.
.
PRISMA Chart Through Different Phases of the Systematic Review. Note. PRISMA: Reporting Items for Systematic Review and Meta-analysis.
Based on the current evaluation, the highest prevalence of infected cases was related to blood group A, which was estimated at 39.06 (95% CI: 36.22-41.94). Although the random effect model revealed high and significant heterogeneity (I2 = 94.67, P < 0.05, Figure 2). Moreover, results related to Egger’s test and funnel plot showed significant publication biases (t = 0.11, P = 0.25, Figure S1, Supplementary File 2).
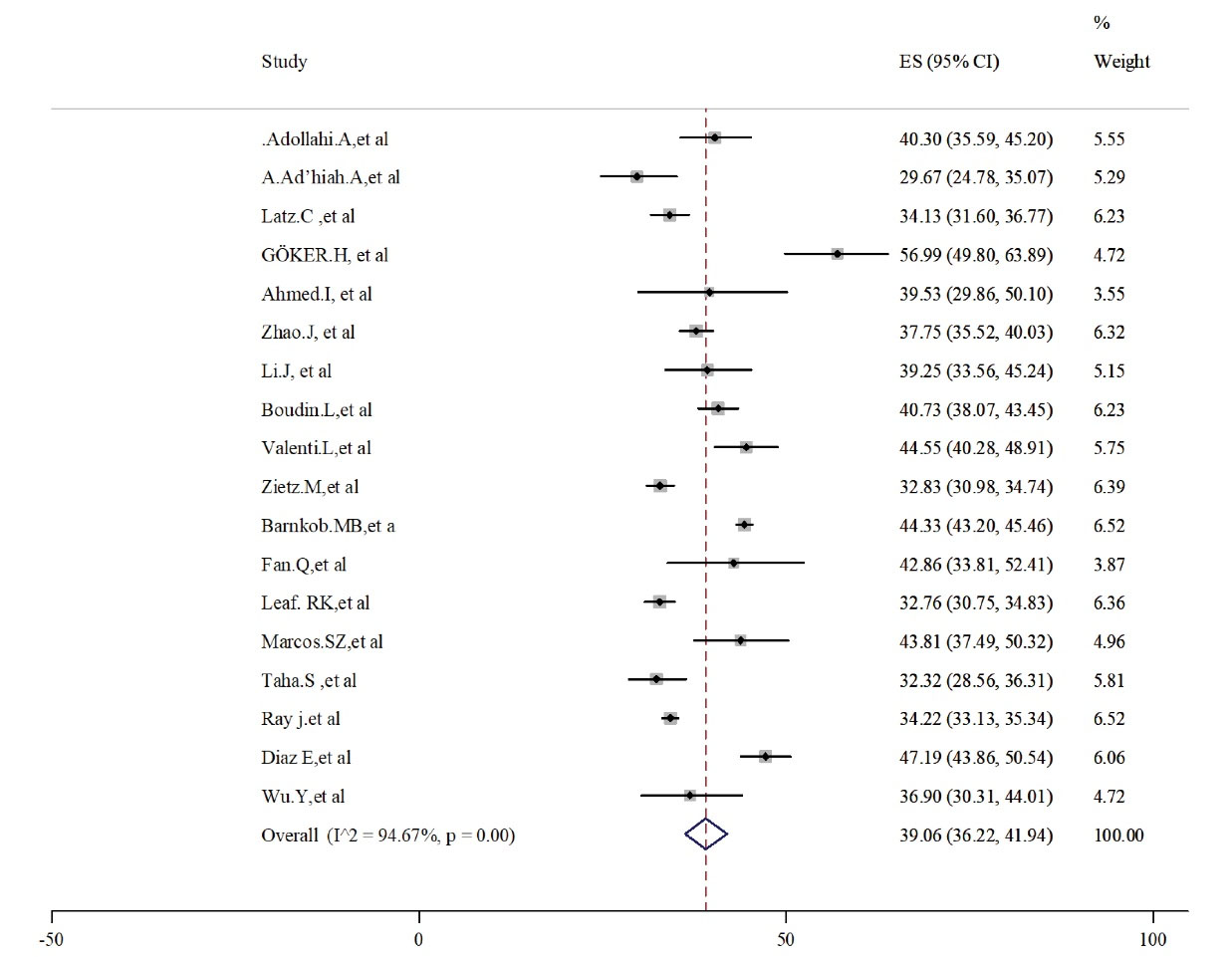
Figure 2.
Prevalence of Blood Group Type A in Alive Patients With COVID-19.
Note. COVID-19: Coronavirus disease 19.
.
Prevalence of Blood Group Type A in Alive Patients With COVID-19.
Note. COVID-19: Coronavirus disease 19.
Blood group O with a prevalence of 35.60 (95% CI: 32.48-38.79) was known as the second predominance in infected patients (Figure 3). High and significant heterogeneity between 17 studies led to the use of the random effect model (I2 = 95.88, P < 0.001). Egger’s test and the related funnel plot confirmed no publication bias (t = 5.77, P < 0.001, Figure S1, Supplementary File 2).
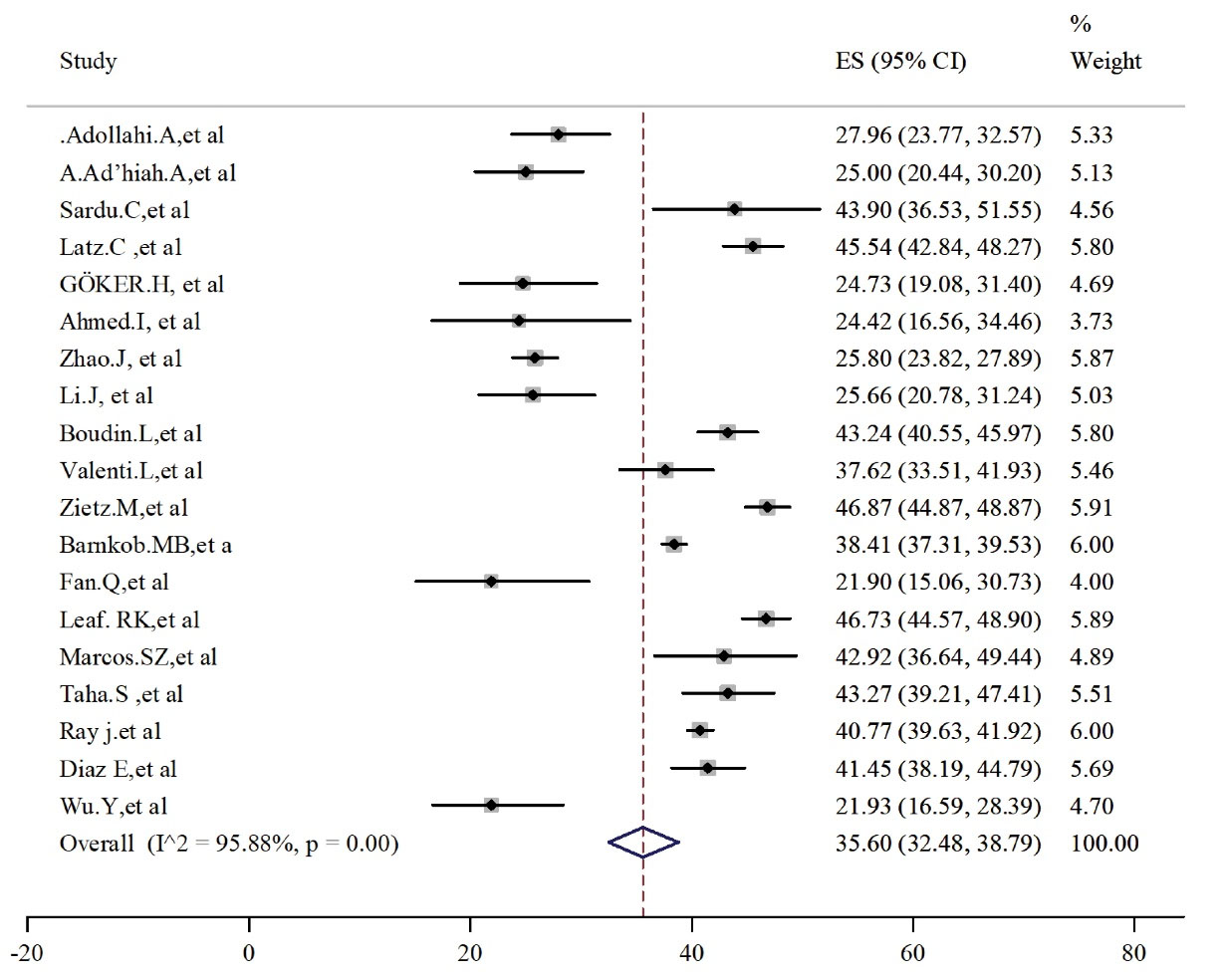
Figure 3.
Prevalence of Blood Group Type O in Alive Patients With COVID-19.
Note. COVID-19: Coronavirus disease 19; CI: Confidence interval.
.
Prevalence of Blood Group Type O in Alive Patients With COVID-19.
Note. COVID-19: Coronavirus disease 19; CI: Confidence interval.
Patients with blood group B and AB were less than the other two groups. The pooled estimated prevalence was 7.72 (95% CI: 5.06-10.88) and 16.23 (95% CI: 12.86-19.91) for AB (Figure 4) and B (Figure 5) groups, respectively. Heterogeneity was high and significant among these analyzed studies. Results for publication bias assessments (Figure S1, Supplementary File 2) were significant for blood type AB (t = 0.35, P = 0.73) but not for blood type B (t = 2.51, P = 0.025).
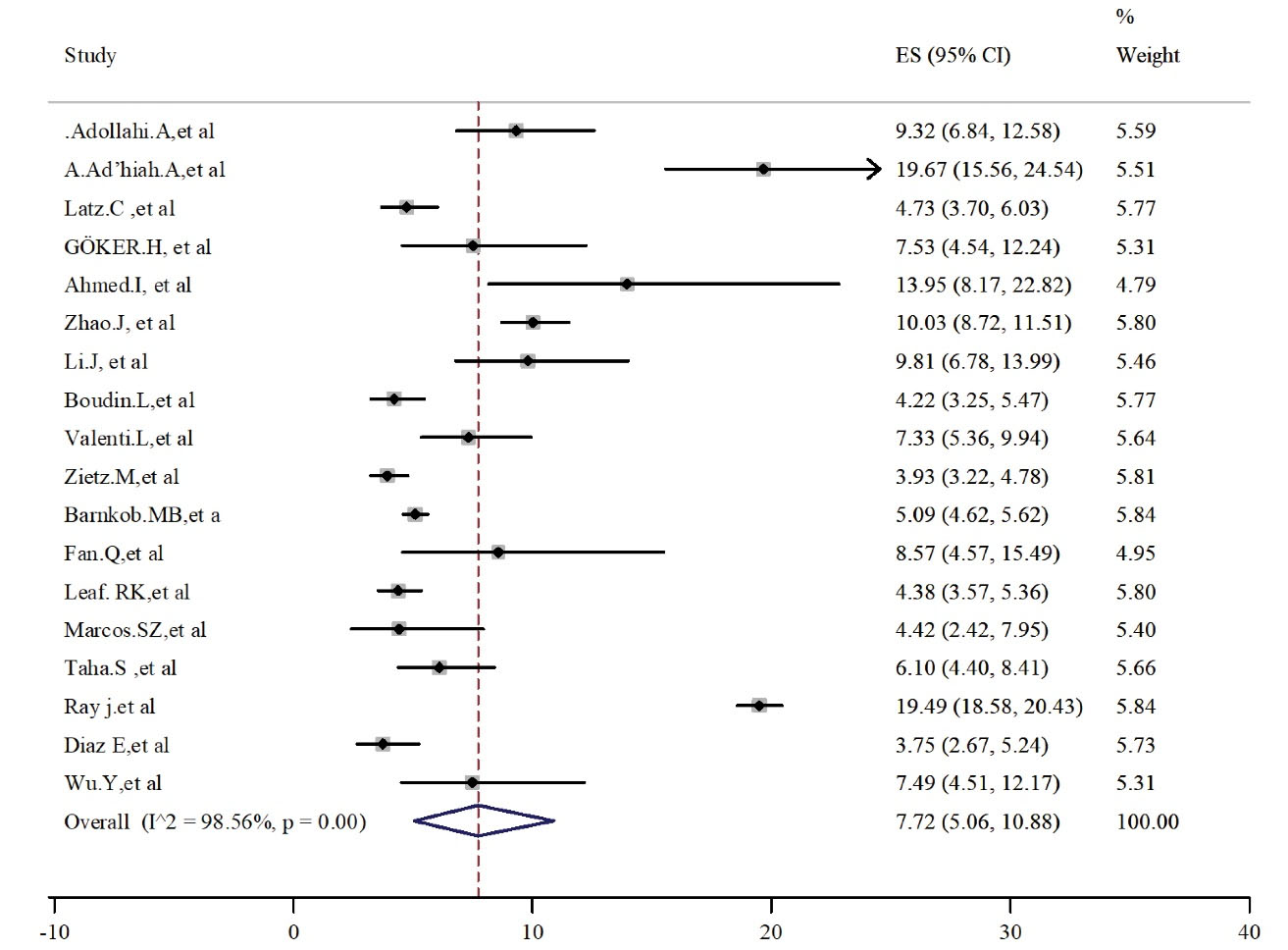
Figure 4.
Prevalence of Blood Group Type AB in Alive Patients With COVID-19.
Note. COVID-19: Coronavirus disease 19; CI: Confidence interval.
.
Prevalence of Blood Group Type AB in Alive Patients With COVID-19.
Note. COVID-19: Coronavirus disease 19; CI: Confidence interval.
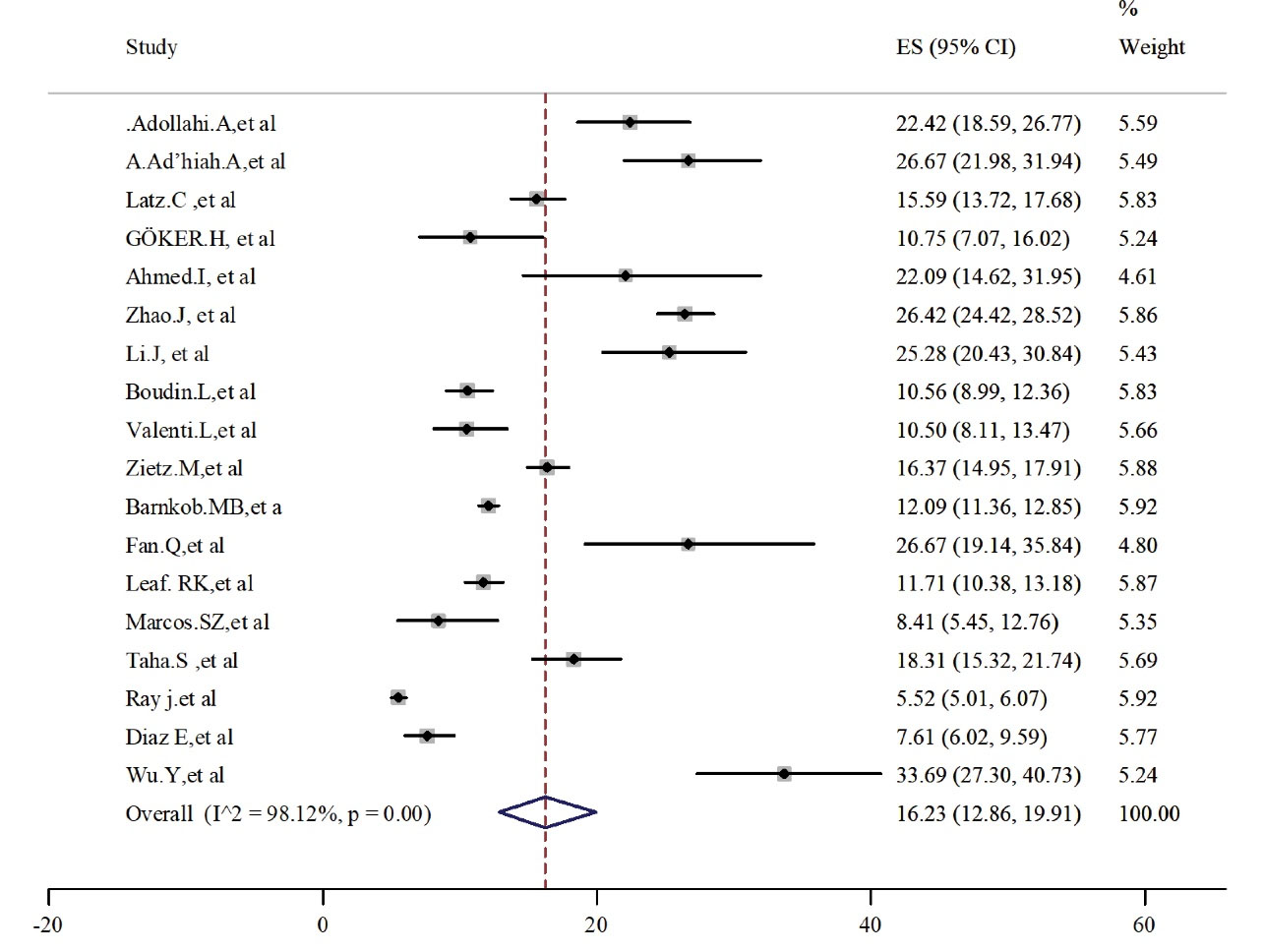
Figure 5.
Prevalence of Blood Group Type B in Alive Patients With COVID-19.
Note. COVID-19: Coronavirus disease 19; CI: Confidence interval.
.
Prevalence of Blood Group Type B in Alive Patients With COVID-19.
Note. COVID-19: Coronavirus disease 19; CI: Confidence interval.
COVID-19. Note. COVID-19: Coronavirus disease 19; CI: Confidence interval.
Subgroup Analysis
To evaluate the most prevalent ABO blood type in the dead patients of COVID-19, a subgroup analysis was performed, and it was found that blood types A and O had the highest prevalence in dead infected cases. More details are provided in Table 2. The funnel plots are depicted in Figure S2 (Supplementary File 2).
Table 2.
Prevalence of ABO Blood Types in Patients Died From COVID-19
|
Blood Group Type
|
Number of Studies
|
Prevalence (95% CI)
|
I
2
|
Heterogeneity Test
|
Egger’s Test
|
|
Z
|
P
Value
|
t
|
P
Value
|
| A |
7 |
39.41 (33.45–45.53) |
83.77 |
36.98 |
< 0.001 |
7.57 |
< 0.001 |
| O |
8 |
32.17 (24.53–40.31) |
91.38 |
81.24 |
< 0.001 |
15 |
< 0.001 |
| AB |
7 |
7.64 (4.80–11.05) |
81.11 |
31.77 |
< 0.001 |
1.15 |
0.3 |
| B |
7 |
16.05 (12.50–19.94) |
74.84 |
23.84 |
< 0.001 |
2.90 |
0.03 |
Note. COVID-19: Coronavirus disease 19; CI: Confidence interval.
Discussion
To decrease the current pandemic’s toll, it is imperative to understand all aspects of infectious disease. As mentioned earlier, this study aimed to identify the most prevalent blood group type to adjust the risk differences between infected patients. Although various case-control studies have focused on determining the association of ABO blood groups with COVID-19, a major concern is considered in terms of the study design. One of these concerns is related to the general population (Control) which is not confirmed with exposure criteria (18,30,31). Moreover, in the included studies, control groups were categorized into the general population, the COVID-19 negative population, and blood donors. The first group included individuals with no history of respiratory infections such as bacterial pneumonia, mycoplasma pneumonia, tuberculosis, and other types of pneumonia, healthy volunteers, registered patients in hospitals before the COVID-19 pandemics (23,26,29) and the second one encompassed patients with no history of the COVID-19 positive test or non-tested people (22,32). Finally, blood donors included healthy individuals who registered in national blood banks (16). Despite this variation in the control groups, they were a sample from a normal population in that country, and using these reports, it was found that case and controls are in a similar range in different blood types. Therefore, caution is required about interpreting these results and making a certain decision. It was necessary to understand the most prevalent ABO blood group in infected patients and manage the disease and related risks. It is necessary to mention that the distribution of ABO blood groups in COVID-19 patients was higher compared to the control or general population in all case-control studies (33), and the proportion and distribution of ABO blood groups in COVID-19 patients and the general population were similar to our study. The interesting point was that among the patients, blood groups A and O were more prevalent in comparison with the other groups. However, it should be careful that the type of the control population did not significantly account for the observed heterogeneity in previous reports (33). The current results revealed that COVID-19 patients contained a higher percentage of cases with types A and O blood groups compared to other types. Moreover, the rate of death was more in patients with blood groups A and O as well. As it became clear that in previous viral pandemic diseases (SARS and MERS) and related studies, host susceptibility can also depend on the expression of blood group antigens (34). Considering that these antigens were expressed by most human cells and tissues, they had a modification role in the immune response against the enveloped viruses, bacteria, and parasite infections and acted as receptors/coreceptors (16). In fact, organizing the membrane microdomains by blood group antigens can assist in intracellular uptake and adhere of microorganisms by the transduction of the signal (35).
Spike (S) is a large transmembrane protein in SARS-CoV-2 which mediates the association of the virus with the angiotensin-converting enzyme-2 (ACE-2) receptor, has a critical role in the viral entrance to cells, and causes infection (36). In different studies during the COVID-19 crisis, it has been confirmed that ACE-2 is the main receptor of the virus and acts as the main host cell surface receptor for virus spike protein. Although this receptor has been expressed on different organs in humans, it has been clarified that different confounders can differ ACE-2 exposure with the virus ligands (37). In other studies, it was mentioned that cases with blood group type A are more susceptible to COVID-19 whereas patients with type O blood group had a lower risk (38,39). Based on the results of Fan et al, low lymphocyte counts in blood group type A, in comparison with other types, may have a key role in the severity of COVID-19 in this blood group; however, lymphopenia is one of the complications in SARS-CoV-2 (23). In an evaluation by Zietz et al, the risk of intubation is more in B and AB blood types while this is in the least possible among A types (21). According to our results, the prevalence and death risk of SARS-CoV-2 are in more ranges in the type A blood group, which is in line with the result of Fan et al, indicating that this may be due to the relation between the natural antibodies of the ABO blood system and the ACE-2 receptor. They mentioned that the ACE-2 receptor is blocked in cases with anti-A antibodies, (Blood groups B and O) and then these individuals are more resistant to COVID-19 compared to the type A blood group (23).
The presence of cardiovascular diseases is another factor which increases the risk of the COVID-19 infection and its severity in communication. In a previous study by Dai, a strong relationship was found between the A allele of ABO blood groups and the increased risk of cardiovascular diseases, implying that patients with type A blood group are more susceptible to cardiovascular diseases and thus further risk of the SARS-CoB-V-2 infection (40). Potential clinical implications about ABO blood groups and other infectious diseases bring the speculations close to a similar pattern to SARS-CoV-2.
Based on the findings of another study, there may be a meaningful relationship between the progression of COVID-19 and ABO polymorphism, which somehow affects the dysregulation of vascular tone, permeability, and the induction of cytokine storm and redox stress. It was shown that ABO polymorphism can affect disease progression by the molecular mechanism that does not involve natural antibodies. This needs more studies in the future during the current crisis (41).
Although our study results confirmed that A and O are the most prevalent blood types in COVID-19 patients, previous reports demonstrated different susceptibility and severity of this infectious disease in different blood groups. These results require further evaluations due to multifactorial confounders and different functions in COVID-19 severity. The potential limitation of our study was the lack of sufficient information about the distribution of underlying diseases in blood groups and evaluating this relationship.
Conclusions
The results of the current meta-analysis showed that A and O blood groups are the most prevalent types in dead and alive patients with COVID-19. However, it must be considered that the genetic changing of the virus during the crisis may cause different patterns of the epidemiology of the disease. Accordingly, it is highly recommended the high-risk blood group types be evaluated during the epidemic time in COVID-19, especially in different geographical areas and races. The findings further represented that types B and AB had the lowest incidence in both groups. Thus, the ABO blood groups and their substances were also expressed in different cell types including respiratory endothelium and gastrointestinal cells, and the results of this kind of study will be helpful in the management of risk factors during this respiratory crisis. Eventually, knowing the risk factors and awareness of the people will assist health policy-makers in controlling the infection and being more careful about health protocols and their risk of suffering the disease.
Authors’ Contribution
AE: Project administration, conceptualization, data curation, and writing the original draft. FJ: Formal analysis and methodology. AA and NP: Visualization, review, and editing.
Conflict of Interests
None.
Ethical Approval
Not applicable.
Funding
None.
Supplementary Materials
Supplementary file 1 contains Table S1 respectively.
(pdf)
Supplementary file 2 contains Figures S1 & S2, respectively.
(pdf)
References
- Albahri OS, Al-Obaidi JR, Zaidan AA, Albahri AS, Zaidan BB, Salih MM. Helping doctors hasten COVID-19 treatment: Towards a rescue framework for the transfusion of best convalescent plasma to the most critical patients based on biological requirements via ml and novel MCDM methods. Comput Methods Programs Biomed 2020; 196:105617. doi: 10.1016/j.cmpb.2020.105617 [Crossref] [ Google Scholar]
- Huang J, Hume AJ, Abo KM, Werder RB, Villacorta-Martin C, Alysandratos KD. SARS-CoV-2 infection of pluripotent stem cell-derived human lung alveolar type 2 cells elicits a rapid epithelial-intrinsic inflammatory response. Cell Stem Cell 2020; 27(6):962-73. doi: 10.1016/j.stem.2020.09.013 [Crossref] [ Google Scholar]
- Singhal T. A review of coronavirus disease-2019 (COVID-19). Indian J Pediatr 2020; 87(4):281-6. doi: 10.1007/s12098-020-03263-6 [Crossref] [ Google Scholar]
- Velavan TP, Meyer CG. Mild versus severe COVID-19: laboratory markers. Int J Infect Dis 2020; 95:304-7. doi: 10.1016/j.ijid.2020.04.061 [Crossref] [ Google Scholar]
- Wilson N, Kvalsvig A, Barnard LT, Baker MG. Case-fatality risk estimates for COVID-19 calculated by using a lag time for fatality. Emerg Infect Dis 2020; 26(6):1339-441. doi: 10.3201/eid2606.200320 [Crossref] [ Google Scholar]
- Javanmardi F, Keshavarzi A, Akbari A, Emami A, Pirbonyeh N. Prevalence of underlying diseases in died cases of COVID-19: a systematic review and meta-analysis. PLoS One 2020; 15(10):e0241265. doi: 10.1371/journal.pone.0241265 [Crossref] [ Google Scholar]
- Mahase E. Coronavirus COVID-19 has killed more people than SARS and MERS combined, despite lower case fatality rate. BMJ 2020; 368:m641. doi: 10.1136/bmj.m641 [Crossref] [ Google Scholar]
- Gérard C, Maggipinto G, Minon JM. COVID-19 and ABO blood group: another viewpoint. Br J Haematol 2020; 190(2):e93-e4. doi: 10.1111/bjh.16884 [Crossref] [ Google Scholar]
- Leaf RK, Al-Samkari H, Brenner SK, Gupta S, Leaf DE. ABO phenotype and death in critically ill patients with COVID-19. Br J Haematol 2020; 190(4):e204-e8. doi: 10.1111/bjh.16984 [Crossref] [ Google Scholar]
- Zhang F, Hughes C. A caution in association of ABO blood group with COVID-19. Glob Clin Transl Res 2020; 2(3):51-3. doi: 10.36316/gcatr.02.0031 [Crossref] [ Google Scholar]
- Wells G. Newcastle-Ottawa Scale. http://www.lri.ca/programs/ceu/oxford.htm. 2007.
- Abdollahi A, Mahmoudi-Aliabadi M, Mehrtash V, Jafarzadeh B, Salehi M. The novel coronavirus SARS-CoV-2 vulnerability association with ABO/Rh blood types. Iran J Pathol 2020; 15(3):156-60. doi: 10.30699/ijp.2020.125135.2367 [Crossref] [ Google Scholar]
- Ad’hiah AH, Allami RH, Mohsin RH, Abdullah MH, Al-Sa’ady AJ, Alsudani MY. Evaluating of the association between ABO blood groups and coronavirus disease 2019 (COVID-19) in Iraqi patients. Egypt J Med Hum Genet 2020; 21(1):50. doi: 10.1186/s43042-020-00097-x [Crossref] [ Google Scholar]
- Sardu C, Marfella R, Maggi P, Messina V, Cirillo P, Codella V. Implications of AB0 blood group in hypertensive patients with COVID-19. BMC Cardiovasc Disord 2020; 20(1):373. doi: 10.1186/s12872-020-01658-z [Crossref] [ Google Scholar]
- Latz CA, DeCarlo C, Boitano L, Png CYM, Patell R, Conrad MF. Blood type and outcomes in patients with COVID-19. Ann Hematol 2020; 99(9):2113-8. doi: 10.1007/s00277-020-04169-1 [Crossref] [ Google Scholar]
- Göker H, Aladağ Karakulak E, Demiroğlu H, Ayaz Ceylan Ç M, Büyükaşik Y, Inkaya A. The effects of blood group types on the risk of COVID-19 infection and its clinical outcome. Turk J Med Sci 2020; 50(4):679-83. doi: 10.3906/sag-2005-395 [Crossref] [ Google Scholar]
- Ahmed I, Quinn L, Tan BK. COVID-19 and the ABO blood group in pregnancy: a tale of two multiethnic cities. Int J Lab Hematol 2021; 43(1):e45-e7. doi: 10.1111/ijlh.13355 [Crossref] [ Google Scholar]
- Zhao J, Yang Y, Huang H, Li D, Gu D, Lu X, et al. Relationship between the ABO blood group and the COVID-19 susceptibility. medRxiv. 2020. 10.1101/2020.03.11.20031096.
- Martins-Filho PR, Tavares CSS, Santos VS. Factors associated with mortality in patients with COVID-19 A quantitative evidence synthesis of clinical and laboratory data. Eur J Intern Med 2020; 76:97-9. doi: 10.1016/j.ejim.2020.04.043 [Crossref] [ Google Scholar]
- Boudin L, Janvier F, Bylicki O, Dutasta F. ABO blood groups are not associated with risk of acquiring the SARS-CoV-2 infection in young adults. Haematologica 2020; 105(12):2841-3. doi: 10.3324/haematol.2020.265066 [Crossref] [ Google Scholar]
- Zietz M, Zucker J, Tatonetti NP. Associations between blood type and COVID-19 infection, intubation, and death. Nat Commun 2020; 11(1):5761. doi: 10.1038/s41467-020-19623-x [Crossref] [ Google Scholar]
- Barnkob MB, Pottegård A, Støvring H, Haunstrup TM, Homburg K, Larsen R. Reduced prevalence of SARS-CoV-2 infection in ABO blood group O. Blood Adv 2020; 4(20):4990-3. doi: 10.1182/bloodadvances.2020002657 [Crossref] [ Google Scholar]
- Fan Q, Zhang W, Li B, Li DJ, Zhang J, Zhao F. Association between ABO blood group system and COVID-19 susceptibility in Wuhan. Front Cell Infect Microbiol 2020; 10:404. doi: 10.3389/fcimb.2020.00404 [Crossref] [ Google Scholar]
- Leaf RK, Al-Samkari H, Brenner SK, Gupta S, Leaf DE. ABO phenotype and death in critically ill patients with COVID-19. Br J Haematol 2020; 190(4):e204-e8. doi: 10.1111/bjh.16984 [Crossref] [ Google Scholar]
- Zalba Marcos S, Antelo ML, Galbete A, Etayo M, Ongay E, García-Erce JA. Infection and thrombosis associated with COVID-19: possible role of the ABO blood group. Med Clin (Barc) 2020; 155(8):340-3. doi: 10.1016/j.medcli.2020.06.020 [Crossref] [ Google Scholar]
- Taha SAH, Osman MEM, Abdoelkarim EAA, Holie MAI, Elbasheir MM, Abuzeid NMK. Individuals with a Rh-positive but not Rh-negative blood group are more vulnerable to SARS-CoV-2 infection: demographics and trend study on COVID-19 cases in Sudan. New Microbes New Infect 2020; 38:100763. doi: 10.1016/j.nmni.2020.100763 [Crossref] [ Google Scholar]
- Muñiz-Diaz E, Llopis J, Parra R, Roig I, Ferrer G, Grifols J. Relationship between the ABO blood group and COVID-19 susceptibility, severity and mortality in two cohorts of patients. Blood Transfus 2021; 19(1):54-63. doi: 10.2450/2020.0256-20 [Crossref] [ Google Scholar]
- Ray JG, Schull MJ, Vermeulen MJ, Park AL. Association between ABO and Rh blood groups and SARS-CoV-2 infection or severe COVID-19 illness: a population-based cohort study. Ann Intern Med 2021; 174(3):308-15. doi: 10.7326/m20-4511 [Crossref] [ Google Scholar]
- Wu Y, Feng Z, Li P, Yu Q. Relationship between ABO blood group distribution and clinical characteristics in patients with COVID-19. Clin Chim Acta 2020; 509:220-3. doi: 10.1016/j.cca.2020.06.026 [Crossref] [ Google Scholar]
- Zhang Y, Garner R, Salehi S, La Rocca M, Duncan D. Association between ABO blood types and coronavirus disease 2019 (COVID-19), genetic associations, and underlying molecular mechanisms: a literature review of 23 studies. Ann Hematol 2021; 100(5):1123-1132. doi: 10.1007/s00277-021-04489-w [Crossref] [ Google Scholar]
- Boudin L, Dutasta F. Relationship between ABO blood groups and coronavirus disease 2019: study design matters. Clin Infect Dis 2021; 72(11):e918. doi: 10.1093/cid/ciaa1473 [Crossref] [ Google Scholar]
- Focosi D, Carla IM, Lanza M. ABO Blood Group Correlations with Covid-19: Cohort Choice Makes A Difference. Clin Infect Dis 2021; 72(11):e919. doi: 10.1093/cid/ciaa1495 [Crossref] [ Google Scholar]
- Golinelli D, Boetto E, Maietti E, Fantini MP. The association between ABO blood group and SARS-CoV-2 infection: a meta-analysis. PLoS One 2020; 15(9):e0239508. doi: 10.1371/journal.pone.0239508 [Crossref] [ Google Scholar]
- Cheng Y, Cheng G, Chui CH, Lau FY, Chan PK, Ng MH. ABO blood group and susceptibility to severe acute respiratory syndrome. JAMA 2005; 293(12):1450-1. doi: 10.1001/jama.293.12.1450-c [Crossref] [ Google Scholar]
- Silva-Filho JC, de Melo CGF, de Oliveira JL. The influence of ABO blood groups on COVID-19 susceptibility and severity: a molecular hypothesis based on carbohydrate-carbohydrate interactions. Med Hypotheses 2020; 144:110155. doi: 10.1016/j.mehy.2020.110155 [Crossref] [ Google Scholar]
- Gemmati D, Tisato V. Genetic hypothesis and pharmacogenetics side of renin-angiotensin-system in COVID-19. Genes (Basel) 2020; 11(9):1044. doi: 10.3390/genes11091044 [Crossref] [ Google Scholar]
- Ni W, Yang X, Yang D, Bao J, Li R, Xiao Y. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit Care 2020; 24(1):422. doi: 10.1186/s13054-020-03120-0 [Crossref] [ Google Scholar]
- Zaidi FZ, Zaidi ARZ, Abdullah SM, Zaidi SZA. COVID-19 and the ABO blood group connection. Transfus Apher Sci 2020; 59(5):102838. doi: 10.1016/j.transci.2020.102838 [Crossref] [ Google Scholar]
- Zalba Marcos S, Antelo ML, Galbete A, Etayo M, Ongay E, García-Erce JA. Infection and thrombosis associated with COVID-19: Possible role of the ABO blood group. Med Clin (Barc) 2020; 155(8):340-3. doi: 10.1016/j.medcli.2020.06.020 [Crossref] [ Google Scholar]
- Dai X. ABO blood group predisposes to COVID-19 severity and cardiovascular diseases. Eur J Prev Cardiol 2020; 27(13):1436-7. doi: 10.1177/2047487320922370 [Crossref] [ Google Scholar]
- Ellinghaus D, Degenhardt F, Bujanda L, Buti M, Albillos A, Invernizzi P. Genomewide association study of severe COVID-19 with respiratory failure. N Engl J Med 2020; 383(16):1522-34. doi: 10.1056/NEJMoa2020283 [Crossref] [ Google Scholar]