Avicenna Journal of Clinical Microbiology and Infection. 8(4):156-163.
doi: 10.34172/ajcmi.2021.28
Review Article
Who Is Immune Against COVID-19 and Safe to Return to Work: The Impact of Laboratory Assays
Ala Habibian 1  , Hoorieh Soleimanjahi 1, *
, Hoorieh Soleimanjahi 1, *  , Taravat Bamdad 1, Seyed Mahmood Seyed Khorrami 1, Atefeh Yari 1
, Taravat Bamdad 1, Seyed Mahmood Seyed Khorrami 1, Atefeh Yari 1
Author information:
1Department of Virology, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran
*
Corresponding author: Hoorieh Soleimanjahi, Ph.D., Department of Virology, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran, Tel/Fax: +98- 2182883561, Email:
soleim_h@modares.ac.ir
Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the cause of coronavirus disease 2019 (COVID-19) as a pandemic infectious disease which has led to thousands of deaths around the world. The Coronaviridae family is the second cause of the common cold that targets human respiratory tracts. Specific diagnostic laboratory tests in addition to clinical investigations would be helpful in confirming COVID-19 in the early stages for controlling the disease. Upon SARS-CoV-2 infection, antibody responses are produced during the early phase of illness (>7 days), meanwhile, viral nucleic acid real-time reverse-transcription polymerase chain reaction (rRT-PCR) test is applied as the confirmatory assay in the first 5-6 days after the onset of illness. Due to the rise of antibodies, the viral nucleic acid represents a gradual decline. These laboratory tests may be considered valuable for monitoring the patient’s status to prevent the spreading of infections and keep him/her in quarantine. The results of molecular and serological assays revealed that whether the person is recovered and protected against disease. Furthermore, regarding the rise of antibody titer and undetectable viral RNA, it may be possible to make a decision about when the recovered people could back to work and social life.
Keywords: Respiratory syndrome, ELISA, RT-PCR, Antibody, Serological assay
Copyright and License Information
© 2021 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Background
The third serious emerging disease from the Coronaviridae family appeared from Wuhan, China in late 2019. This recent disease was officially called coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Two previous coronaviruses included severe acute respiratory syndrome coronavirus (SARS-CoV) and the Middle East respiratory syndrome (MERS-CoV) that appeared in 2003 and 2014, respectively (1,2). Considering that COVID-19 was extremely spread worldwide, the World Health Organization (WHO) announced a pandemic stance on March 11, 2020 (3,4). After a month interval from the defined pandemic situations, there are currently more than 3 million infected people globally confirmed, and more than 210 thousand deaths occurred during this time (5).
The case fatality rate (CFR) is the death rate which is calculated by the percentage of the proportion of individuals who die due to a certain disease per total infected cases in a specified period of time. The CFR for MERS (34.4%) and SARS (~10%) was estimated to be more compared to COVID-19. According to the WHO data, CFR for COVID-19 was 3.7% at the beginning of the pandemic, but numerous amounts of infected cases have recently revealed that CFR is surpassed and has reached 6.9% (6-10).
The estimated median time for the incubation period in COVID-19 is 4.8 and 5.5 days, inside and outside mainland China, respectively. Although the incubation time of COVID-19 is totally variable, even may lasting two weeks, but the mean time of it is around 5 days. (7,11). Li et al determined that COVID-19 is transmissible at the end of the incubation period, and although COVID-19 infectivity may be considerable during this time, it is still obscure (12). Isolation and restriction in contacts should be performed to break the chain of disease transmission and reduce the chance of exposure to prevent disastrous outcomes. The generation of secondary infections could be managed by this strategy and contact tracing. This may decrease the basic reproductive number (R0) (13,14), which demonstrates the ability of the virus to transmit an infectious disease from a single infected person to new cases in a naïve population. In this parameter, previous exposure immunity is not participated, including vaccination history and other interventions. SARS-CoV-2 is an important concern for all societies around the world and R0 is a major factor for estimating the rate of transmission of COVID-19. The amount of R0 will be controlled by social distance and quarantine since an interpretation of R0 < 1 is due to contact limitation and the reduction of newly infected cases (14-17).
SARS-CoV-2 is diagnosed by some in vitro diagnostic tests based on either serological immune assay (IA) or molecular assays such as the real-time reverse transcription-polymerase chain reaction (rRT-PCR). Several methods including enzyme-linked immunosorbent assay, chemiluminescence IA (CLIA), and lateral flow IA exist to perform serologic IA in order to detect produced immunoglobulin M (IgM) and immunoglobulin G (IgG) by the patient during the infection. rRT-PCR is currently a reference laboratory test for the etiologic diagnosis of COVID-19. The marvelous time for collecting specimens with a high viral load is in 5-6 days after the onset of symptoms. In contrast to rRT-PCR, the specimens for serological tests must be collected around 10 days post-onset of illness. Thus, in the acute phase of the disease or asymptomatic infections, a combination of serological and molecular assays must be performed to control this infectious disease (18-21). This review aimed to investigate and compare the results of the serological and molecular assays of different studies to find the value of laboratory tests results to make decisions whether employments are safe for returning to work.
Virus Features
SARS-CoV-2 is classified within the genus of beta-coronavirus, allocated to the Coronaviridae family and in the order of Nidoviral. SARS-CoV-2 can cause severe acute respiratory syndrome-related coronavirus the same as two other members of this genus, namely, SARS-CoV and MERS-CoV (1,22). Similar to other viruses in this genus, SARS-CoV-2 contains four structural proteins. Three of four cases are located in the surface of the viral membrane and named spike glycoprotein (S), envelop protein (E), and membrane protein (M). The next one, nucleocapsid protein (N) is put in the proximity of the virus genome. In addition to these structural proteins, there are several non-structural proteins which are primarily translated into two sets of polyproteins (pp1a & pp1b) and then processed to mature proteins including ribonucleic acid (RNA)-dependent RNA polymerase (RdRp), papain-like protease (PLpro), 3-chymotrypsin-like protease (3CLpro), helicase, and the like autoproteolytically (23,24), the details of which are depicted in Figure 1A.

Figure 1.
Structure and Genome Organization of SARS-CoV-2. Note. ORF: Open reading frame; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; FA: Schematic showing the major structural proteins of coronavirus virion. B: Schematic image of the complete genome organization of SARS-CoV-2 showing the replicase gene included two overlapping ORF (pp1a and pp1b); basic genes (S, E, M, & N) and accessory genes.
.
Structure and Genome Organization of SARS-CoV-2. Note. ORF: Open reading frame; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; FA: Schematic showing the major structural proteins of coronavirus virion. B: Schematic image of the complete genome organization of SARS-CoV-2 showing the replicase gene included two overlapping ORF (pp1a and pp1b); basic genes (S, E, M, & N) and accessory genes.
SARS-CoV-2 applies the S protein as a receptor to enter host cells. This transmembrane protein (~180 kDa) is composed of S homo-trimer molecules on the surface of the virus and cleaved to S1 as an attachment subunit and S2 which are critical for the fusion of the virus. Considering that S1 is involved in receptor binding domains, SARS-CoV-2 could recognize angiotensin-converting enzyme 2 as cellular host receptors on the respiratory tract and attach strongly. Further, S glycoprotein is the main target for generating neutralization antibodies (18,25,26).
The most abundant structural protein in coronaviruses is M protein, which is responsible for the formation of the viral envelope. Furthermore, M protein is related to other structural proteins of coronavirus for organizing the assembly process. In addition, this protein is fairly conserved among coronaviruses in a similar genus (23,27).
The pentameric integral membrane protein, which is called E protein, is moderately small (~12 kDa) and not glycosylated, and is reported to act as an ion channel and plays an important role in viral infectivity (23,28).
In addition to the surficial mentioned proteins, there is another structural one which binds to the RNA genome of coronavirus, the so-called N protein. This phosphoprotein may increase the affinity of the protein to viral RNA in comparison with non-viral RNA. It was revealed that N protein may provide the accurate and rapid diagnosis of COVID-19 by antigenicity features on the linear epitopes of the outer surface protein (23,29).
These structural proteins are commonly expressed from one-third near the 3’ end of the genome. Moreover, two-thirds of the genome in the 5’ position consists of two large open reading frames including rep1a and rep1b for producing 16 non-structural proteins from polyproteins which have been mentioned in previous studies (23,30), the related data of which are illustrated in Figure 1B.
SARS-CoV-2 Molecular Assay
Acute respiratory distress syndrome and velocity spread of new coronavirus led to the crucial request for assays that are accurate tests and have the ability to quickly diagnose COVID-19. For this purpose, several RT-PCR assays were designed to detect the virus and measure the viral load to determine disease diagnosis (31,32). The RT-PCR is an appropriate and common test for detecting infectious diseases such as COVID-19 by the detection of SARS-CoV-2 nucleic acid from the bronchoalveolar lavage (BAL) fluid, sputum, nasopharyngeal, and oropharyngeal swabs of respiratory specimens and blood and anal swabs (33).
Briefly, viral SARS-CoV-2 RNA is isolated from the respiratory tract specimens of patients. The reverse transcriptase process is performed following the extraction and purification of the RNA genome and used as a template to turn into complementary deoxyribonucleic acid (cDNA) due to specific viral primers. Afterward, the synthesized cDNA can be used as a template, along with specifically designed primers and probes to amplify the target sequences. Positive results are detected by the cleavage of the reporter on the probe and amplification of fluorescence enhancement. However, the accurate results of this technique may strongly depend on the onset time of symptoms and the specimens and routes of sampling (34,35). Furthermore, successive amplification of the viral genome in the suspected patient can be interpreted as a positive result when the cycle threshold value raises in > 40 cycles, otherwise, the result may be considered as negative (36).
According to the literature and the comparison between different experimental techniques (i.e., nucleic acid amplification test, immunoassay, and rapid tests), the RT-PCR is recommended by the WHO and Centers for Disease Control and Prevention (CDC) as the most sensitive and specific assay for improving diagnosis in laboratories for COVID-19 in order to reduce the spread of the infection (37,38).
In a study released in January 2020, two monoplex quantitative RT-PCR (qRT-PCR) assays were developed according to the WHO protocol and the two highly conserved regions of the involved viral RNA genome (i.e., orf1b and N). This experiment was evaluated using a panel for controls in which the spectrum of human coronaviruses, other respiratory viruses, and the sputum specimen of healthy individuals were used as the negative control. According to the results, it was recommended that RT-PCR on N and S regions are reliable for screening and confirmation, respectively. Additionally, it was determined that the N region is more sensitive regarding RT-PCR assay for SARS-CoV-2 detection (39,40). The other study was performed in China on a cluster of the family who traveled to Wuhan from their city, Shenzhen, Guangdong province, China. Two members of this family visited a hospital in Wuhan and were infected with a new coronavirus and transmitted it to others by close contact. In this study, Chan et al designed an RT-PCR assay on two separate regions of coronavirus genomic RNA as the highly conserved region (RdRp) and a highly variable region in the S protein. All respiratory specimens of this family cluster were positive for RdRp and the S region by RT-PCR assay except for the specimen that was collected on the 9th day of the disease onset (41).
Furthermore, other molecular targets are located on the genomic RNA of coronaviruses, including regions that belong to non-structural proteins, along with four structural proteins. The main point is that at least, a couple of molecular targets should be selected to prevent cross-sectional reactions in this assay by other seasonal coronaviruses. In this regard, there are two moderately different guidelines designed by the WHO and CDC, as the WHO recommended the E region for screening and following the RdRp region for confirmation and CDC suggested two nucleocapsid protein targets (36,42). Corman et al demonstrated the high sensitivity for E, RdRp, and the N region with 5.2, 3.8, and 3.2 RNA copies per reaction at 95% detection probability for RT-PCR assay, respectively (37).
Principally, the consequences of both analytical and pre-analytical factors of rRT-PCR may report false results. The misclassification rate depends on pre-analytical including sample quality (poor specimens transport condition and sample collection technique) and the sampling time point (disease status). The rRT-PCR negative results must be interpreted while considering both relevant background data including clinical manifestations and the epidemiological history of suspected patients. In this situation, 24-hour repeated sampling is required and might display fabulous results (21,36).
Determination of Immunity to COVID-19 by Serologic Tests
Serological assays are based on antibody production against viral antigens among the structural proteins, especially S and N proteins. These assays provide a method to determine the number of infected individuals, whose sensitivity strongly relies on the phase of the disease. Certainly, seroconversion in most patients did not occur during the incubation period or the onset of illness (36,43).
COVID-19 infection is typically divided into three phases. The first phase is the incubation and non-severe phase in which individuals are found relatively asymptomatic with or without detectable new infections. The second phase is non-severe symptomatic and rapid growth in the cases of infection with the presence of the virus. The final phase is the severe acute respiratory symptom in which highly viral genomes are observable. Previous research showed that the levels of IgM antibodies were detectable in both symptomatic and non-severe symptomatic patients 5 days after the onset of illness (44).
Jin et al performed a retrospective study was performed in China on 43 COVID-19 patients through CLIA. All patients were previously confirmed by laboratory tests. The median age was 47 years (ranging from 7 to 74), and 33 suspected persons were enrolled in this study as the control group (the median age was 31 years). In the condition of tested positive for the virus by molecular detection, 27 patients of the COVID-19 group were investigated for viral antibodies with a 16-day interval from the onset of symptoms to the first serological test. Among 27 patients, 13 and 24 cases were IgM (48.1%) and IgG (88.9%) positive, respectively. Those three residual patients were negative for both IgM and IgG. Initially, IgM tends to increase during the first days after the disease onset while it will be diminished during the time. On the other hand, the IgG rate increases following a rise in IgM and remains permanent over time. This study revealed that after being virus-negative by the two oral swabs with 24-hour intervals, IgG-positive titers become double and are significantly different before virus-negative tests (45), which contradict the results of the study by Zhang et al, demonstrating that molecular assay on an oral swab in early stages was positive whereas in the late stages of the disease, the anal swab was positive and the oral swab was negative. Meanwhile, IgM and IgG positive titers increased from 50% to 81% and 81% to 100%, respectively. Thus, other routes may include the samples of the anatomical sites of COVID-19 transmission except for the molecular detection of the virus in respiratory samples, and more importantly, serological tests would be strongly valuable to confirm disease exclusion (46).
In another study, Zhao et al reported the advantage of antibodies for distinguishing COVID-19. This study was performed on 173 patients by 535 sequential plasma samples collected since their admission to the hospital. All samples were tested for total antibodies (Ab), along with IgM and IgG antibodies against SARS-CoV-2 in addition to RT-PCR for determining the RNA rate by 0-7, 8-14, and 15-39 days after the onset of illness. During the first week of illness, the rate of RNA was estimated at around 66.7% (58/87) among the collected specimens. Nonetheless, in the late phase of illness and during 15-39 days post-onset of the disease, the positive rate of RNA was evaluated at about 45.5% (25/55). On the other hand, there were 100% (30/30), 93.3% (28/30), and 73.3% (22/30) positive amounts of total antibody, IgM, and IgG in patients with undetectable RNA, respectively. Finally, they suggested the high sensitivity of Ab in comparison to IgM and IgG for detecting SARS-CoV-2 infection. Moreover, they revealed the value of IgM for determining either the acute disease or post-epidemic area during the next epidemic occurrence. Conversely, the total antibody and IgG are functional for epidemiological studies (47).
Likewise, Grzelak et al assessed neutralizing antibodies by four different serological tests on N and S proteins and found that among 51 severe symptomatic hospitalized patients, seroconversion was detectable between 5 and 14 days after the onset of illness. In fact, the median time of seroconversion for IgM was 5-12 and 14 days for IgA and IgG, respectively. Furthermore, among 175 mild symptomatic patients who were included in this study, neutralizing antibodies were detected 10-15 days post-onset of illness. The findings of this study confirmed the correlation between the rate and appearance of neutralizing antibodies with the severity of disease symptoms. Additionally, more sensitive assays may detect mild symptomatic patients and asymptomatic persons by weaker responses (48). In another study, three types of antibodies (i.e., IgA, IgM, and IgG) were recognized among 216 sera samples from 87 confirmed COVID-19 patients during 41 days through CLIA on both N and receptor binding domains of the S region. The highest median concentration of IgA was 8.8 μg/mL during 16-21 days after the onset of symptoms, and then it started to reduce to 3.6 μg/mL on day 41. IgM reached its peak amount of concentration (7.25 μg/mL) while its sensitivity was low among other types of antibodies. The highest median concentration of IgG was 16.5 μg/mL and later than others (during 21-25 days after the onset of illness) and remained approximately 11.4 μg/mL on day 41. There was a remarkable correlation between the concentration of IgA and the severity of symptoms (49).
Interestingly, Guo et al followed up a group of healthcare workers during the SARS outbreak during 2004-2015. They concluded that the IgG level against the whole virus (around 60%) was significantly high in this cohort study for 12 years in comparison with the IgG rate against N protein (around 30%). Their results showed that the IgG level remains persistently and determined the IgG value to the vaccine developer to design a strategy either for SARS-CoV or SARS-CoV-2 (50).
In the cohort study, Wu et al followed up patients with specified history were followed up during 1265 days and demonstrated that IgM and IgG reached the maximum amount of around 21-30 days and 2-4 months after the onset of illness, respectively. After 60 days post-onset of illness, the median of the optical density of IgM decreased and reached the cut-off point. The same condition occurred for IgG one year after the onset of symptoms (51). Similarly, there are other similar findings with variable sample sizes and follow-up durations for indicating the convalescence of the SARS coronavirus neutralizing antibody (52,53). Müller et al analyzed the sero convalescent of the MERS coronavirus in nearly 13 provinces of Saudi Arabia among three different groups in general populations (n=1, 009), camel shepherds (n=87), and people working in slaughterhouses (n=140) and reported that MERS-coronavirus antibodies were positive in 0.15%, 2.3%, and 3.6%, respectively. It seems that the low antibody titers may not be protective against subsequent MERS coronavirus infections (54,55).
Discussion and Conclusion
Now, we encounter with a newly emerging disease of the Coronaviridae family which is intensely spreading worldwide. Although China in Asia was the center of this pandemic, other 212 countries and territories including Asian, European, and North and South American countries have encountered with this emergency health situation (18). There are considerable numbers of asymptomatic cases for COVID-19 which have been reported to transmit illness. Indeed, some quantities of recovered patients or asymptomatic people may be a carrier of illness causing a problem in the health policy system (56-58). Molecular diagnostic assay (the RT-PCR) was primarily used to detect and screen infectious and then to confirm COVID-19 due to the absence of the serological test for this new virus (59). Considerably, serological methods are helpful in confirmation and cases where the rRT-PCR is negatively false due to the pre-analytic (poor sampling) or analytic process and a decline in the viral load as a result of antibody production (36). Moreover, the rRT-PCR assay maybe beneficial for asymptomatic patients since Liu et al identified no magnificent differences in IgM and IgG production between the confirmed (83%) and suspected (78.8%) patients. Suspected patients negative for viral RNA could be distinguished by performing the rRT-PCR assay to determine the load of viral RNA in the proper time (60). Thus, for beneficial analysis, a combination of serological and molecular assays should be performed to determine the status of COVID-19 according to the seroconversion and presence of genomic RNA (47,61), the related data are displayed in Figure 2.
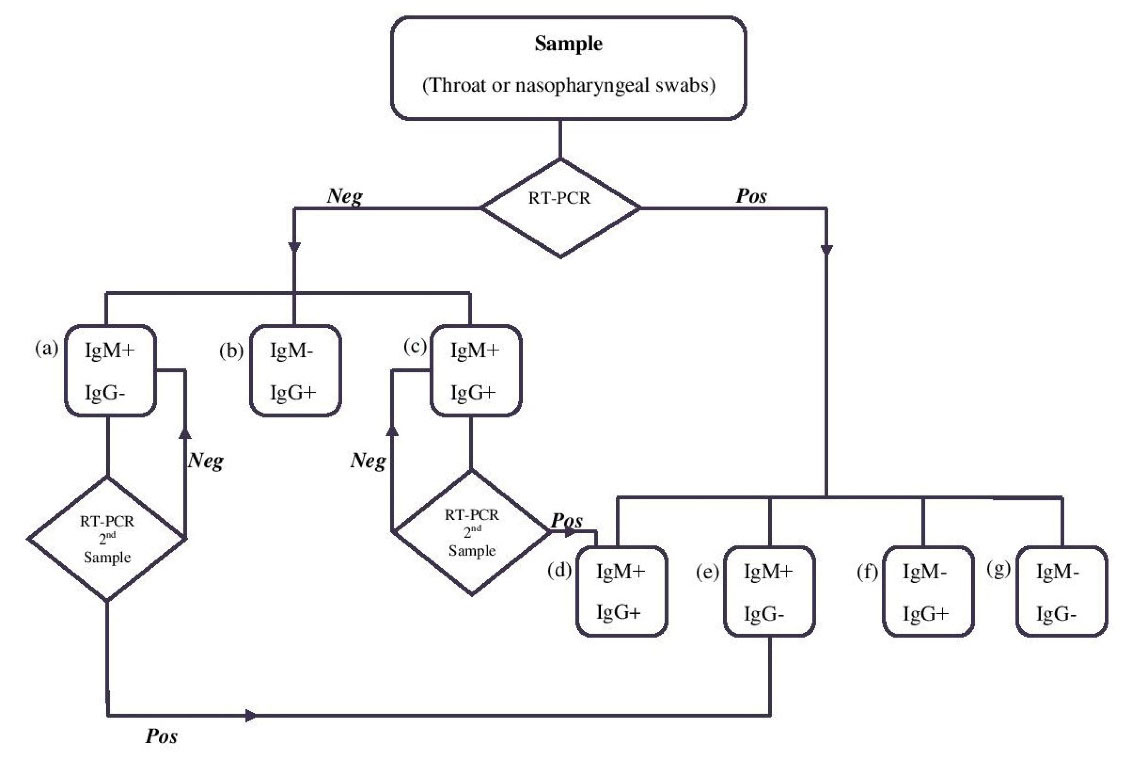
Figure 2.
Algorithm for the Assessment of SARS-CoV-2 in COVID-19 Patients. Note. IgM: Immunoglobulin M; RT-PCR: Real-time polymerase chain reaction; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; COVID: Coronavirus disease. The algorithm displays a procedure which begins with RT-PCR detection. A RT-PCR positive result for the RT-PCR test confirms COVID-19 illness. The detection of antibodies in combination with RT-PCR would be helpful in managing the spreading of the disease. (a): Suspected primary infection; (b): Past infection: Immune; (c): Maybe in the recovery phase: The 2nd sample should be taken immediately for RT-PCR confirmatory; (d): Active phase of infection, these carrier individuals should remain in quarantine; (e): Early stage of infection: These carrier individuals should remain in quarantine; (f): Late stage of infection: These carrier individuals should remain in quarantine; (g): Window period: These carrier individuals should remain in quarantine.
.
Algorithm for the Assessment of SARS-CoV-2 in COVID-19 Patients. Note. IgM: Immunoglobulin M; RT-PCR: Real-time polymerase chain reaction; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; COVID: Coronavirus disease. The algorithm displays a procedure which begins with RT-PCR detection. A RT-PCR positive result for the RT-PCR test confirms COVID-19 illness. The detection of antibodies in combination with RT-PCR would be helpful in managing the spreading of the disease. (a): Suspected primary infection; (b): Past infection: Immune; (c): Maybe in the recovery phase: The 2nd sample should be taken immediately for RT-PCR confirmatory; (d): Active phase of infection, these carrier individuals should remain in quarantine; (e): Early stage of infection: These carrier individuals should remain in quarantine; (f): Late stage of infection: These carrier individuals should remain in quarantine; (g): Window period: These carrier individuals should remain in quarantine.
In spite of nasopharyngeal and oropharyngeal swabs which have been recommended because of their tolerability for patients, sputum, and BAL are more suitable samples from the lower respiratory tracts for efficiently detecting the viral load. Considerably, during the first 5-6 days of illness, the increased viral load is due to their presence in respiratory tracts (36,41,62). According to Wu et al, neutralizing antibodies were detected before 10 days of the illness onset although the titer was low. The titers increased from 10 to 15 days and stayed constant. They also indicated that older patients have a higher titer of neutralizing antibodies in comparison with young ones. Moreover, the level of C-reactive protein in aging patients was high in contrast with the count of lymphocytes, which is low in their blood assay (63).
As it is known, two different categorized tests are performing to indicate whether admitted COVID-19 patients become immune or not. It is extremely important to conduct a precise test at the right time. Considering that there is no specific therapy or vaccine for this new virus, the result of laboratory assays is strongly important to decide to continue quarantine in order to prevent the spread of the virus. One research study revealed that SARS-CoV-2 remains constant even more than 60 days post-onset of illness. Indeed, viral shedding may continue even after seroconversion and recovery from the disease (64).
Furthermore, Wu et al indicated that the new coronavirus is disabled to induce neutralizing antibodies against SARS-CoV. Moreover, Amanat et al reported that there is no cross-reactivity between coronavirus-NL63 and SARS-CoV-2. However, another report by Guo et al demonstrated strong cross-reactivity between the positive plasma of SARS-CoV patients and the nucleoprotein of SARS-CoV-2 by the western blot. They also concluded that there is no cross-reactivity between the new coronavirus and previous coronaviruses causing the common cold, including OC43, NL63, 229E, and HKU-1 (61,63,65).
Thus, making a decision for when and how to return to work and restart social activities is a crucial subject that has remained unanswered accurately. Although experiments revealed that viral clearance may occur by two sequential negative RT-PCR (for a pair of the region) and IgG neutralizing antibody titer, it should be considered that we may occasionally encounter with false-negative or false-positive results. Although cross-reactivity is more unlikely, there are limited studies that have noted this characteristic as mentioned previously. The question arises regarding when a person is positive for both antibody and RT-PCR, whether be protective against COVID-19 or not, or whether this extracted RNA belongs to the productive virus and it can replicate or not. However, it is obviously understandable that the RT-PCR negative, along with positive antibodies defines the reduced chance of the viral spread and may be protective for the person. On the other hand, there were several recovered people who rebounded by COVID-19 symptoms. Actually, this group of patients may have previously recovered from COVID-19 by producing low levels of neutralizing antibodies (63).
Definitively, the network involving laboratorians, clinicians, epidemiologists, and social scientists should manage and make a decision whether to continue the quarantine and when and under which circumstances people may back to work and common life.
To further help discover truths and reveal secrets, several types of research should be performed in the near future. Since there were several recovered people who rebounded by COVID-19 symptoms, the mechanisms of humoral and cellular immunity behind this illness should be investigated to determine the permanent protectivity level in the recovered patients. Moreover, investigating the production of passive immunity in order to protect against COVID-19 as an infectious disease is of necessity.
Acknowledgements
We gratefully thank the Research Deputy of Tarbiat Modares University for the support and contribution to conducting this study.
Conflict of Interests
The authors have no conflict of interests to declare that are relevant to the content of this article.
Ethical Approval
Not applicable.
References
- de Wit E, van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol 2016; 14(8):523-34. doi: 10.1038/nrmicro.2016.81 [Crossref] [ Google Scholar]
- Wu Y, Ho W, Huang Y, Jin DY, Li S, Liu SL. SARS-CoV-2 is an appropriate name for the new coronavirus. Lancet 2020; 395(10228):949-50. doi: 10.1016/s0140-6736(20)30557-2 [Crossref] [ Google Scholar]
- Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, et al. The feasibility of convalescent plasma therapy in severe COVID- 19 patients: a pilot study. medRxiv. 2020. 10.1101/2020.03.16.20036145
- WHO. [Online].; 2020 [cited 2020 March 11. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen.
- WHO. [Online].; 2020 [cited 2020 April 11. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
- Wu F, Wang A, Liu M, Wang Q, Chen J, Xia S, et al. Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications. medRxiv. 2020. 10.1101/2020.03.30.20047365
- Park M, Thwaites RS, Openshaw PJM. COVID‐19: lessons from SARS and MERS. Eur J Immunol 2020; 50(3):308-11. doi: 10.1002/eji.202070035 [Crossref] [ Google Scholar]
- Spychalski P, Błażyńska-Spychalska A, Kobiela J. Estimating case fatality rates of COVID-19. Lancet Infect Dis 2020; 20(7):774-5. doi: 10.1016/s1473-3099(20)30246-2 [Crossref] [ Google Scholar]
- Ritchie H, Roser M. Our World in Data. [Online].; 2020 [cited 2020 March 25. Available from: https://ourworldindata.org/covid-mortality-risk.
- Jiang S, Hillyer C, Du L. Neutralizing antibodies against SARS-CoV-2 and other human coronaviruses. Trends Immunol 2020; 41(5):355-9. doi: 10.1016/j.it.2020.03.007 [Crossref] [ Google Scholar]
- Baum SG, Sa L. NEJM journal watch. [Online]. [cited 2020 March 13. Available from: https://www.jwatch.org/na51083/2020/03/13/covid-19-incubation-period-update.
- Li P, Fu JB, Li KF, Liu JN, Wang HL, Liu LJ. Transmission of COVID-19 in the terminal stages of the incubation period: a familial cluster. Int J Infect Dis 2020; 96:452-3. doi: 10.1016/j.ijid.2020.03.027 [Crossref] [ Google Scholar]
- Kucharski AJ, Russell TW, Diamond C, Liu Y, Edmunds J, Funk S. Early dynamics of transmission and control of COVID-19: a mathematical modelling study. Lancet Infect Dis 2020; 20(5):553-8. doi: 10.1016/s1473-3099(20)30144-4 [Crossref] [ Google Scholar]
- Hellewell J, Abbott S, Gimma A, Bosse NI, Jarvis CI, Russell TW. Feasibility of controlling COVID-19 outbreaks by isolation of cases and contacts. Lancet Glob Health 2020; 8(4):e488-e96. doi: 10.1016/s2214-109x(20)30074-7 [Crossref] [ Google Scholar]
- Yuan J, Li M, Lv G, Lu ZK. Monitoring transmissibility and mortality of COVID-19 in Europe. Int J Infect Dis 2020; 95:311-5. doi: 10.1016/j.ijid.2020.03.050 [Crossref] [ Google Scholar]
- Liu Y, Gayle AA, Wilder-Smith A, Rocklöv J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Travel Med 2020; 27(2):taaa021. doi: 10.1093/jtm/taaa021 [Crossref] [ Google Scholar]
- Sahafizadeh E, Sartoli S. Estimating the reproduction number of COVID-19 in Iran using epidemic modeling. medRxiv. 2020. 10.1101/2020.03.20.20038422
- Vashist SK. In vitro diagnostic assays for COVID-19: recent advances and emerging trends. Diagnostics (Basel) 2020; 10(4):202. doi: 10.3390/diagnostics10040202 [Crossref] [ Google Scholar]
- Long QX, Deng HJ, Chen J, Hu JL, Liu BZ, Liao P, et al. Antibody responses to SARS-CoV-2 in COVID-19 patients: the perspective application of serological tests in clinical practice. medRxiv. 2020. 10.1101/2020.03.18.20038018
- Lin D, Liu L, Zhang M, Hu Y, Yang Q, Guo J. Evaluations of the serological test in the diagnosis of 2019 novel coronavirus (SARS-CoV-2) infections during the COVID-19 outbreak. Eur J Clin Microbiol Infect Dis 2020; 39(12):2271-7. doi: 10.1007/s10096-020-03978-6 [Crossref] [ Google Scholar]
- Green K, Graziadio S, Turner P, Fanshawe T, Allen J. Molecular and Antibody Point-of-Care Tests to Support the Screening, Diagnosis and Monitoring of COVID-19. University of Oxford; 2020.
- Gorbalenya AE, Baker SC, Baric RS, De Groot RJ, Drosten C, Gulyaeva AA. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol 2020; 5(4):536-44. doi: 10.1038/s41564-020-0695-z [Crossref] [ Google Scholar]
- Knipe DM, Howley PM, eds. Fields virology. Philadelphia: Lippincott Williams & Wilkins; 2013. p. 825-58.
- Wu C, Liu Y, Yang Y, Zhang P, Zhong W, Wang Y. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm Sin B 2020; 10(5):766-88. doi: 10.1016/j.apsb.2020.02.008 [Crossref] [ Google Scholar]
- Ou X, Liu Y, Lei X, Li P, Mi D, Ren L. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun 2020; 11(1):1620. doi: 10.1038/s41467-020-15562-9 [Crossref] [ Google Scholar]
- Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020; 367(6485):1444-8. doi: 10.1126/science.abb2762 [Crossref] [ Google Scholar]
- Schoeman D, Fielding BC. Coronavirus envelope protein: current knowledge. Virol J 2019; 16(1):69. doi: 10.1186/s12985-019-1182-0 [Crossref] [ Google Scholar]
- Gupta MK, Vemula S, Donde R, Gouda G, Behera L, Vadde R. In-silico approaches to detect inhibitors of the human severe acute respiratory syndrome coronavirus envelope protein ion channel. J Biomol Struct Dyn 2021; 39(7):2617-27. doi: 10.1080/07391102.2020.1751300 [Crossref] [ Google Scholar]
- Diao B, Wen K, Chen J, Liu Y, Yuan Z, Han C, et al. Diagnosis of acute respiratory syndrome coronavirus 2 infection by detection of nucleocapsid protein. medRxiv. 2020. 10.1101/2020.03.07.20032524
- Mirza MU, Froeyen M. Structural elucidation of SARS-CoV-2 vital proteins: computational methods reveal potential drug candidates against main protease, Nsp12 polymerase and Nsp13 helicase. J Pharm Anal 2020; 10(4):320-8. doi: 10.1016/j.jpha.2020.04.008 [Crossref] [ Google Scholar]
- Vogels CBF, Brito AF, Wyllie AL, Fauver JR, Ott IM, Kalinich CC. Analytical sensitivity and efficiency comparisons of SARS-CoV-2 RT-qPCR primer-probe sets. Nat Microbiol 2020; 5(10):1299-305. doi: 10.1038/s41564-020-0761-6 [Crossref] [ Google Scholar]
- Cao X. COVID-19: immunopathology and its implications for therapy. Nature Rev Immunol 2020; 20(5):269-70. doi: 10.1038/s41577-020-0308-3 [Crossref] [ Google Scholar]
- FDA. [Online].; 2020 [cited 2020. Available from: https://www.fda.gov/media/136231/download.
- Zhu J, Rivera K, Baron D. Noisy pooled PCR for virus testing. medRxiv. 2020. 10.1101/2020.04.06.20055384
- Wikramaratna PS, Paton RS, Ghafari M, Lourenço J. Estimating the false-negative test probability of SARS-CoV-2 by RT-PCR. Euro Surveill 2020; 25(50). doi: 10.2807/1560-7917.es.2020.25.50.2000568 [Crossref]
- Tang YW, Schmitz JE, Persing DH, Stratton CW. Laboratory diagnosis of COVID-19: current issues and challenges. J Clin Microbiol 2020; 58(6):e00512-20. doi: 10.1128/jcm.00512-20 [Crossref] [ Google Scholar]
- Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill 2020; 25(3):2000045. doi: 10.2807/1560-7917.es.2020.25.3.2000045 [Crossref] [ Google Scholar]
- Chan JF, Yip CC, To KK, Tang TH, Wong SC, Leung KH. Improved molecular diagnosis of COVID-19 by the novel, highly sensitive and specific COVID-19-RdRp/Hel real-time reverse transcription-PCR assay validated in vitro and with clinical specimens. J Clin Microbiol 2020; 58(5):e00310-20. doi: 10.1128/jcm.00310-20 [Crossref] [ Google Scholar]
- WHO. [Online].; 2020 [cited 2020]. Available from: https://www.who.int/docs/default-source/coronaviruse/peiris-protocol-16-1-20.pdf?sfvrsn=af1aac73_4.
- Chu DKW, Pan Y, Cheng SMS, Hui KPY, Krishnan P, Liu Y. Molecular diagnosis of a novel coronavirus (2019-nCoV) causing an outbreak of pneumonia. Clin Chem 2020; 66(4):549-55. doi: 10.1093/clinchem/hvaa029 [Crossref] [ Google Scholar]
- Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 2020; 395(10223):514-23. doi: 10.1016/s0140-6736(20)30154-9 [Crossref] [ Google Scholar]
- FDA. [Online]. [cited 2020 March]. Available from: https://www.fda.gov/media/134922/download.
- Chen X, Zhou B, Li M, Liang X, Wang H, Yang G. Serology of severe acute respiratory syndrome: implications for surveillance and outcome. J Infect Dis 2004; 189(7):1158-63. doi: 10.1086/380397 [Crossref] [ Google Scholar]
- Shi Y, Wang Y, Shao C, Huang J, Gan J, Huang X. COVID-19 infection: the perspectives on immune responses. Cell Death Differ 2020; 27(5):1451-4. doi: 10.1038/s41418-020-0530-3 [Crossref] [ Google Scholar]
- Jin Y, Wang M, Zuo Z, Fan C, Ye F, Cai Z. Diagnostic value and dynamic variance of serum antibody in coronavirus disease 2019. Int J Infect Dis 2020; 94:49-52. doi: 10.1016/j.ijid.2020.03.065 [Crossref] [ Google Scholar]
- Zhang W, Du RH, Li B, Zheng XS, Yang XL, Hu B. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect 2020; 9(1):386-9. doi: 10.1080/22221751.2020.1729071 [Crossref] [ Google Scholar]
- Zhao J, Yuan Q, Wang H, Liu W, Liao X, Su Y. Antibody responses to SARS-CoV-2 in patients with novel coronavirus disease 2019. Clin Infect Dis 2020; 71(16):2027-34. doi: 10.1093/cid/ciaa344 [Crossref] [ Google Scholar]
- Grzelak L, Temmam S, Planchais C, Demeret C, Tondeur L, Huon C. A comparison of four serological assays for detecting anti-SARS-CoV-2 antibodies in human serum samples from different populations. Sci Transl Med 2020; 12(559):eabc3103. doi: 10.1126/scitranslmed.abc3103 [Crossref] [ Google Scholar]
- Ma H, Zeng W, He H, Zhao D, Jiang D, Zhou P. Serum IgA, IgM, and IgG responses in COVID-19. Cell Mol Immunol 2020; 17(7):773-5. doi: 10.1038/s41423-020-0474-z [Crossref] [ Google Scholar]
- Guo X, Guo Z, Duan C, Chen Z, Wang G, Lu Y, et al. Long-term persistence of IgG antibodies in SARS-CoV infected healthcare workers. medRxiv. 2020. 10.1101/2020.02.12.20021386
- Wu LP, Wang NC, Chang YH, Tian XY, Na DY, Zhang LY. Duration of antibody responses after severe acute respiratory syndrome. Emerg Infect Dis 2007; 13(10):1562-4. doi: 10.3201/eid1310.070576 [Crossref] [ Google Scholar]
- Liu W, Fontanet A, Zhang PH, Zhan L, Xin ZT, Baril L. Two-year prospective study of the humoral immune response of patients with severe acute respiratory syndrome. J Infect Dis 2006; 193(6):792-5. doi: 10.1086/500469 [Crossref] [ Google Scholar]
- Nie Y, Wang G, Shi X, Zhang H, Qiu Y, He Z. Neutralizing antibodies in patients with severe acute respiratory syndrome-associated coronavirus infection. J Infect Dis 2004; 190(6):1119-26. doi: 10.1086/423286 [Crossref] [ Google Scholar]
- Müller MA, Meyer B, Corman VM, Al-Masri M, Turkestani A, Ritz D. Presence of Middle East respiratory syndrome coronavirus antibodies in Saudi Arabia: a nationwide, cross-sectional, serological study. Lancet Infect Dis 2015; 15(5):559-64. doi: 10.1016/s1473-3099(15)70090-3 [Crossref] [ Google Scholar]
- Arabi YM, Hajeer AH, Luke T, Raviprakash K, Balkhy H, Johani S. Feasibility of using convalescent plasma immunotherapy for MERS-CoV infection, Saudi Arabia. Emerg Infect Dis 2016; 22(9):1554-61. doi: 10.3201/eid2209.151164 [Crossref] [ Google Scholar]
- Black JRM, Bailey C, Przewrocka J, Dijkstra KK, Swanton C. COVID-19: the case for health-care worker screening to prevent hospital transmission. Lancet 2020; 395(10234):1418-20. doi: 10.1016/s0140-6736(20)30917-x [Crossref] [ Google Scholar]
- Day M. COVID-19: four fifths of cases are asymptomatic, China figures indicate. BMJ 2020; 369:m1375. doi: 10.1136/bmj.m1375 [Crossref] [ Google Scholar]
- Lan L, Xu D, Ye G, Xia C, Wang S, Li Y. Positive RT-PCR test results in patients recovered from COVID-19. JAMA 2020; 323(15):1502-3. doi: 10.1001/jama.2020.2783 [Crossref] [ Google Scholar]
- Li M, Jin R, Peng Y, Wang C, Ren W, Lv F, et al. Generation of antibodies against COVID-19 virus for development of diagnostic tools. medRxiv. 2020:2020.02.20.20025999. 10.1101/2020.02.20.20025999
- Liu L, Liu W, Zheng Y, Jiang X, Kou G, Ding J. A preliminary study on serological assay for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in 238 admitted hospital patients. Microbes Infect 2020; 22(4-5):206-11. doi: 10.1016/j.micinf.2020.05.008 [Crossref] [ Google Scholar]
- Amanat F, Stadlbauer D, Strohmeier S, Nguyen THO, Chromikova V, McMahon M. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat Med 2020; 26(7):1033-6. doi: 10.1038/s41591-020-0913-5 [Crossref] [ Google Scholar]
- Yu F, Yan L, Wang N, Yang S, Wang L, Tang Y. Quantitative detection and viral load analysis of SARS-CoV-2 in infected patients. Clin Infect Dis 2020; 71(15):793-8. doi: 10.1093/cid/ciaa345 [Crossref] [ Google Scholar]
- Wu F, Wang A, Liu M, Wang Q, Chen J, Xia S, et al. Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications. medRxiv. 2020. 10.1101/2020.03.30.20047365
- Liu WD, Chang SY, Wang JT, Tsai MJ, Hung CC, Hsu CL. Prolonged virus shedding even after seroconversion in a patient with COVID-19. J Infect 2020; 81(2):318-56. doi: 10.1016/j.jinf.2020.03.063 [Crossref] [ Google Scholar]
- Guo L, Ren L, Yang S, Xiao M, Chang D, Yang F. Profiling early humoral response to diagnose novel coronavirus Disease (COVID-19). Clin Infect Dis 2020; 71(15):778-85. doi: 10.1093/cid/ciaa310 [Crossref] [ Google Scholar]