Avicenna Journal of Clinical Microbiology and Infection. 7(3):85-89.
doi: 10.34172/ajcmi.2020.19
Original Article
A Retrospective Phylogenetic Analysis of Matrix Gene and Amantadine Resistance in Avian Influenza (H9N2 subtype) During 2014-2015 in Isfahan, Iran
Majid Gholami-Ahangaran 1, *  , Maziar Haj Salehi 2, Maryam Karimi-Dehkordi 1, Shahrzad Azizi 3
, Maziar Haj Salehi 2, Maryam Karimi-Dehkordi 1, Shahrzad Azizi 3
Author information:
1Department of Clinical Sciences, Faculty of Veterinary Medicine, Shahrekord Branch, Islamic Azad University, Shahrekord, Iran.
2Graduated of Veterinary Medicine Faulty, Shahrekord Branch, Islamic Azad University, Shahrekord, Iran.
3Department of Pathobiology, Faculty of Veterinary Medicine, Shahid Bahonar University of Kerman, Kerman, Iran.
*
Corresponding author: Majid Gholami-Ahangaran, Department of Poultry Diseases, Faculty of Veterinary Medicine, Shahrekord Branch, Islamic Azad University, P.O. Box:166, Shahrekord, IRAN, Email:
mgholami6@gmail.com
Abstract
Background: Influenza is a main viral disease in poultry production that causes various annual economic losses to the poultry production industry. Avian influenza virus (AIV) is susceptible to antigenic changes, and the genome of this virus codes different proteins some of which have more biological properties. The matrix (M) protein is one of these proteins that plays a role in the immunization and pathogenesis of the virus. Therefore, the evaluation of molecular characteristics and changes in the influenza gene can provide a new horizon for further genomic studies. Accordingly, in this study, the molecular characteristics of AI H9N2 strains were compared with those of other reference strains in the world gene bank by determining their M gene sequence.
Methods: In this regard, 4 strains of AIV (H9N2) were selected for the analysis of the M gene sequence. The polymerase chain reaction product was sequenced after its purification from the gel and the amplification of the M gene. Finally, the nucleotide sequence of these strains and other reference strains were aligned and analyzed by MegAlign software using the Clustal W method.
Results: The results indicated that the M gene sequences of AIVs belonging to the last decade were highly similar to each other and other reference strains in special regions such as the ionic gate and the cleavage site. Based on the M sequence, 3 strains appeared to be resistant to amantadine. These viruses in the epitope regions showed a high similarity to the highly pathogenic avian influenza (HPAI) Hong Kong H5N1 strain.
Conclusions: In general, it seems that the sequence of the M gene in Iranian H9N2 strains belonging to the last decade is relatively constant although the continuous monitoring of changes in various genes of the influenza virus is necessary.
Keywords: Avian influenza, M gene, Gene sequence analysis, Iran
Copyright and License Information
© 2020 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Background
Avian influenza (AI) is one of the most important viral and respiratory diseases in the poultry industry, which causes annual economic losses all over the world. The avian influenza virus (AIV) belongs to the Orthomyxovirus family. One of the most prominent properties of this virus is the mutation and recombination that occur by slow and continuous genomic and antigenic changes that have led to the formation of different subtypes (1). In addition, influenza A can cause acute respiratory disease in birds and some mammals such as humans. Further, the viruses of this type of influenza infecting poultry can be divided into two distinct groups based on their biological and virological components and the ability to cause disease. Furthermore, the very virulent viruses cause highly pathogenic avian influenza (HPAI), in which mortality may be as high as 100%. These viruses have been restricted to H5 and H7 subtypes although not all viruses of these subtypes cause HPAI. Moreover, all other viruses cause an extremely milder, primarily respiratory disease, which may be exacerbated by other infections or environmental factors (2). The H9N2 strain of AIV has spread to Iran since June 1998. Although the pathogenicity of this virus is determined according to the biological test and the sequencing of the surface antigen of hemagglutinin and classified to low pathogenic AIV, it has caused various economic losses to the poultry industry since its introduction to Iran due to the high mortality and reductions in production (3). The genome of the influenza virus is fragmented and consists of eight distinct monocular RNA molecules with negative polarity (1). According to (4), studies on the antigenic diversity of this virus in Iran have focused on shifted antigen changes in both 4 and 6 RNA sequences that code the surface antigens of hemagglutinin and neuraminidase. Thus, little information is available about the antigenic diversity of other RNAs encoding other proteins. The matrix gene (M) of the influenza virus consists of 1027 nucleotides, which include two subunits of M1 (nucleotide sequences 26-784) and M2 (26-51 and 740-1007). These components contain 252 and 97 amino acids, respectively. Considering that the M protein involves (coded by RNA part 7) in the mechanism of the immunization and pathogenicity of AIV (5,6), the evaluation of molecular characteristics and changes in this gene can draw a new horizon for future genomic studies. In this regard, the rapid and sensitive real-time polymerase chain reaction (RT-PCR) method was utilized to detect AIV infection based on M gene amplification. More precisely, in this study, the molecular characteristics of AI H9N2 strains were compared with those of other reference strains in the world gene bank by identifying their M gene sequence.
Materials and Methods
To this end, four allantoic fluids of H9N2 AIV strains from a previous study were used to amplify the M gene (2). The initial tracheal and lung samples were collected from dead broiler chickens with severe respiratory symptoms and belonging to 2014 and 2015 from Isfahan province. Then, the RNA was extracted from the allantoic fluid of specific pathogen-free embryonated chicken eggs using the RNA purification kit (Roche, Germany). Next, the RT-PCR was performed to amplify a fragment of the AIV M gene. The applied primer in this study is described in Table 1. The length of the amplified fragment was about 1027 bp of the M gene (7).
Table 1.
The Characteristics of the Applied Primer for Amplifying the M Gene of Avian Influenza
|
Primer Name
|
Primer Sequence
|
Start-Stop
|
Annealing Temperature ( ̊ C)
|
Accession Number
|
Amplified Fragment Length (bp)
|
Target Gene
|
| Bm-M-1 (F) |
F:5’AGCAAAAGCAGGTAG3’ |
1-15 |
45.50 |
NC-007367.1 |
1027 |
M |
| Bm-M-1027R (R) |
R:5’AGTAGAAACAAGGTAGTTTTT3’ |
1027-1007 |
50.50 |
In addition, the RT-PCR was conducted using 10 μL RT-PCR (with magnesium chloride), 2.5 μL dithiothreitol, 1 μL deoxyribonucleotide triphosphate, 2 μL forward primer, 2 μL reverse primer (10 pmol each primer), 1 μL enzyme mix, 4 μL template RNA, and sterilized distilled water (27.5 μL) in the final volume of 50 μL, followed by the reverse transcription of RNA.
The cDNA synthesis was done at 45°C for 45 minutes, primary denaturation at 94°C for 3 minutes, denaturation at 94°C for 60 seconds, annealing at 48°C for 60 seconds, extension at 68°C for 60 seconds, and the final extension at 68°C for 10 minutes. In this study, the process of denaturation, annealing, and extension was repeated for 35 cycles.
Further, a 100-bp marker was prepared from Fermentas Company (Germany), and the final amplified product was detected and analyzed by electrophoresis in 1% agarose gel stained with ethidium bromide.
The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the housekeeping gene. Furthermore, a pair of primers was synthesized by Bioneer (South Korea) for the amplification of the GAPDH gene. The primer sequences for chicken GAPDH were: forward primer, 5’-GGTGGTGCTAAGCGTGTTA-3’; reverse primer, 5’-CCCTCCACAATGCCAA-3’, resulting in an amplified product of 179 bp (Accession No. X01578) according to (8).
Then, the PCR product belonging to the H9N2 strains of AIV was sequenced to analyze the nucleotide and amino acid sequences of the M gene. For this purpose, PCR products were purified with a commercial DNA purification kit (Roche, Germany) from the agarose gel according to the manufacturer’s instruction. The extracted product together with the general primers of the AIV and the specific applied M gene in PCR were sent to MWG-Biotech Company (Germany). Next, strain sequencing was performed directly (Direct automated cycle sequencing) and bilaterally (9).
To analyze the sequence, first, the nucleotide sequence of the M gene was compared to the sequence of other viruses registered in the gene bank using the Basic Local Alignment Search Tool (BLAST) in the EMBL/GenBank gene (WWW.NCBI.nlm.nih.gov/BLAST). After identifying the similarity of sequences with other gene bank viruses, reference strains were selected for further analysis. The sequence of the strains was analyzed with the DNAStar software package (DNAStar Inc., Madison, WI, USA) and then edited using the Edit Sequence software to match the length of the sequences of all strains. Next, nucleotide sequences were translated into the corresponding amino acid sequence in order to increase validity. Eventually, nucleotide and amino acid sequences were aligned and analyzed by MegAlign software (version 5) using the Clustal W method (9).
Results
The comparison of nucleotide and amino acid sequences of recent H9N2 AIV strains (i.e., IR-203, IR-205, IR-208, and IR-209) indicated that these strains most closely correlate with the previous AIV strains of Iran and there is more than 90% similarity in each case. In addition, recent strains are most similar to strain A/Parakeet/Chib /1/97 (H9N2), which varies from 96 to 99%.
Additionally, the analysis of the amino acid sequence in epitope regions located at the M1 protein suggested that the similarity of recent strains in the epitope regions of the M1 protein is above 96%. This similarity reaches 100% in the epitope areas 2 and 3 (141-89). Further, the amino acid sequences of 101-105, as one of the epitope regions located at M1 protein, in all studied sequences are 101-KKLKR-105. In addition, glutamine-methionine is the cleavage site in the M1 protein in all examined strains at positions 164 and 165. The deduced amino acid is constant and histidine in the membrane portion (TM) of the M2 protein at position 37 and all studied strains, respectively.
Moreover, comparing the amino acid sequence of recent strains in positions 26, 27, 30, 31, and 34 of the M2 protein (determining the resistance to amantadine) showed that three strains at position 31 had only S31N replacement (serine to asparagine substitution), the related data of which are depicted in Figure 1. Likewise, the nucleotide sequence of these three strains in the position of 93 M2 genes suggested the thiamine to cytosine substitution (Figure 2).The condition of recent Iranian AIVs in the phylogenetic tree is displayed in Figure 3.
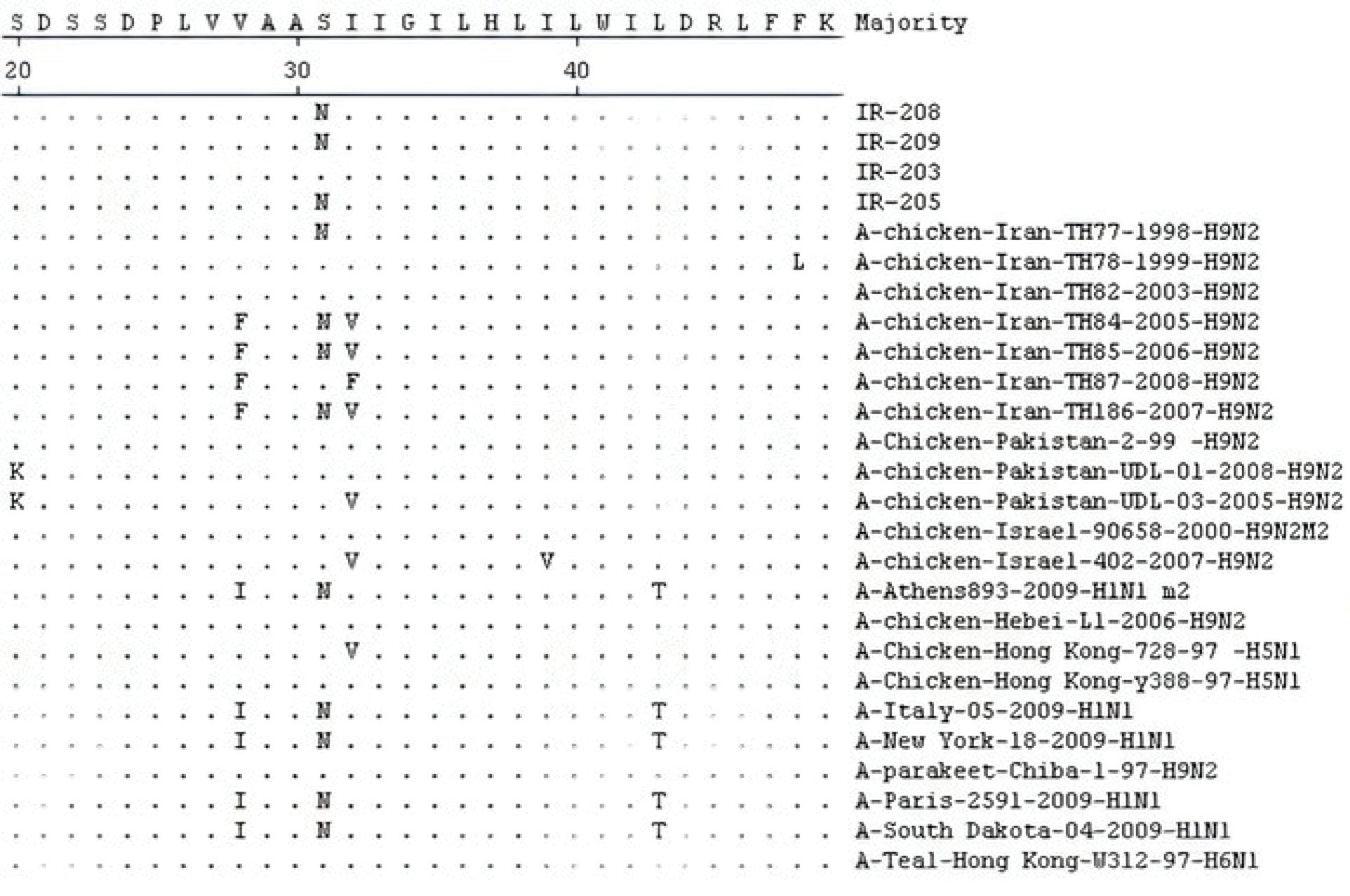
Figure 1.
The Alignment of Deduced Amino Acid Sequence of the M2 Protein of Four H9N2 AIVs (IR-203, IR-205, IR-208 and IR-209) in Comparison to other Reference Strains in World Gene Bank.
.
The Alignment of Deduced Amino Acid Sequence of the M2 Protein of Four H9N2 AIVs (IR-203, IR-205, IR-208 and IR-209) in Comparison to other Reference Strains in World Gene Bank.
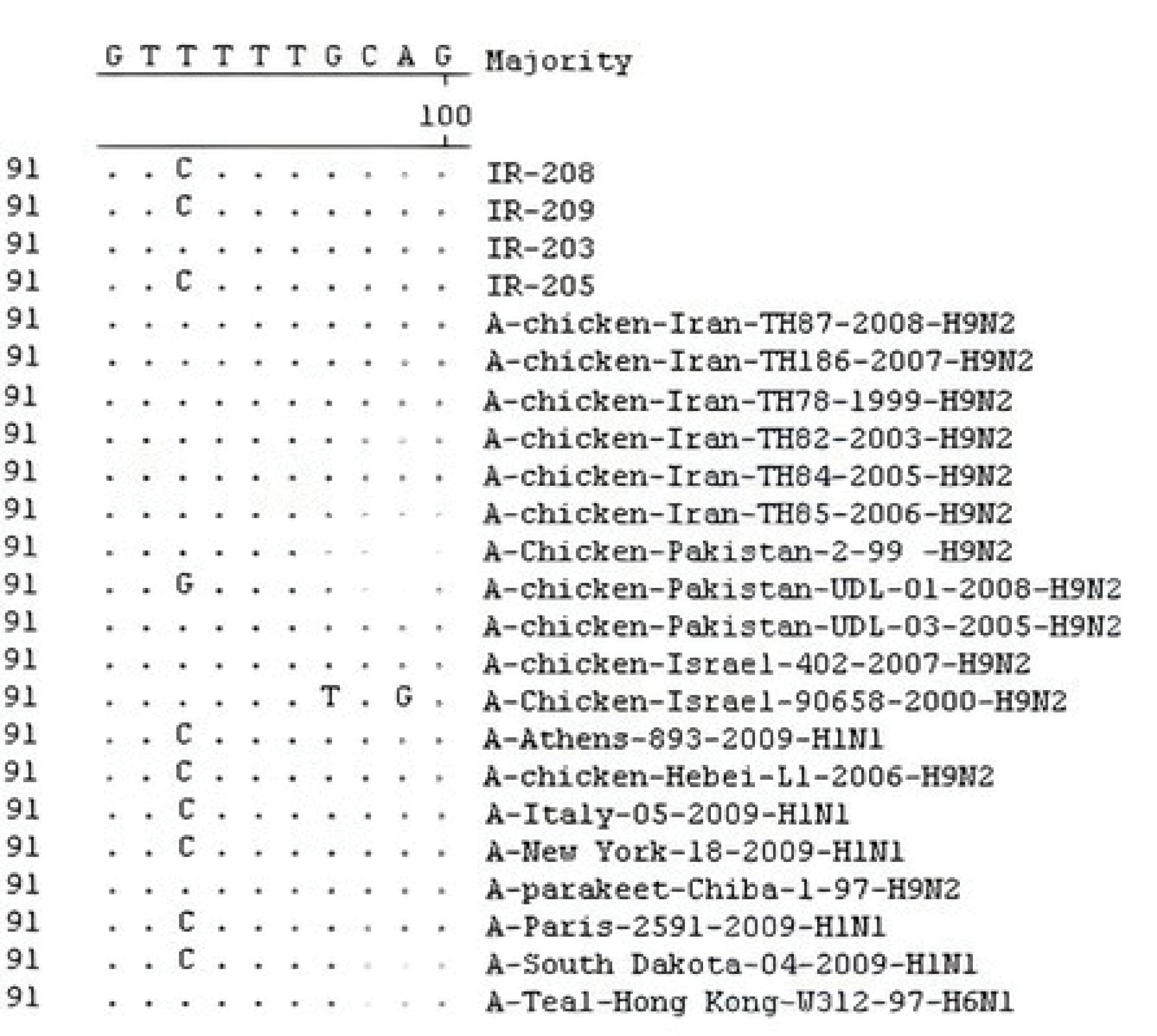
Figure 2.
The Alignment of M2 Gene Sequence of H9N2 AIVs (IR-203, IR-205, IR-208 and IR-209) Compared to Other Strains Registered in the World Gene Bank.
.
The Alignment of M2 Gene Sequence of H9N2 AIVs (IR-203, IR-205, IR-208 and IR-209) Compared to Other Strains Registered in the World Gene Bank.
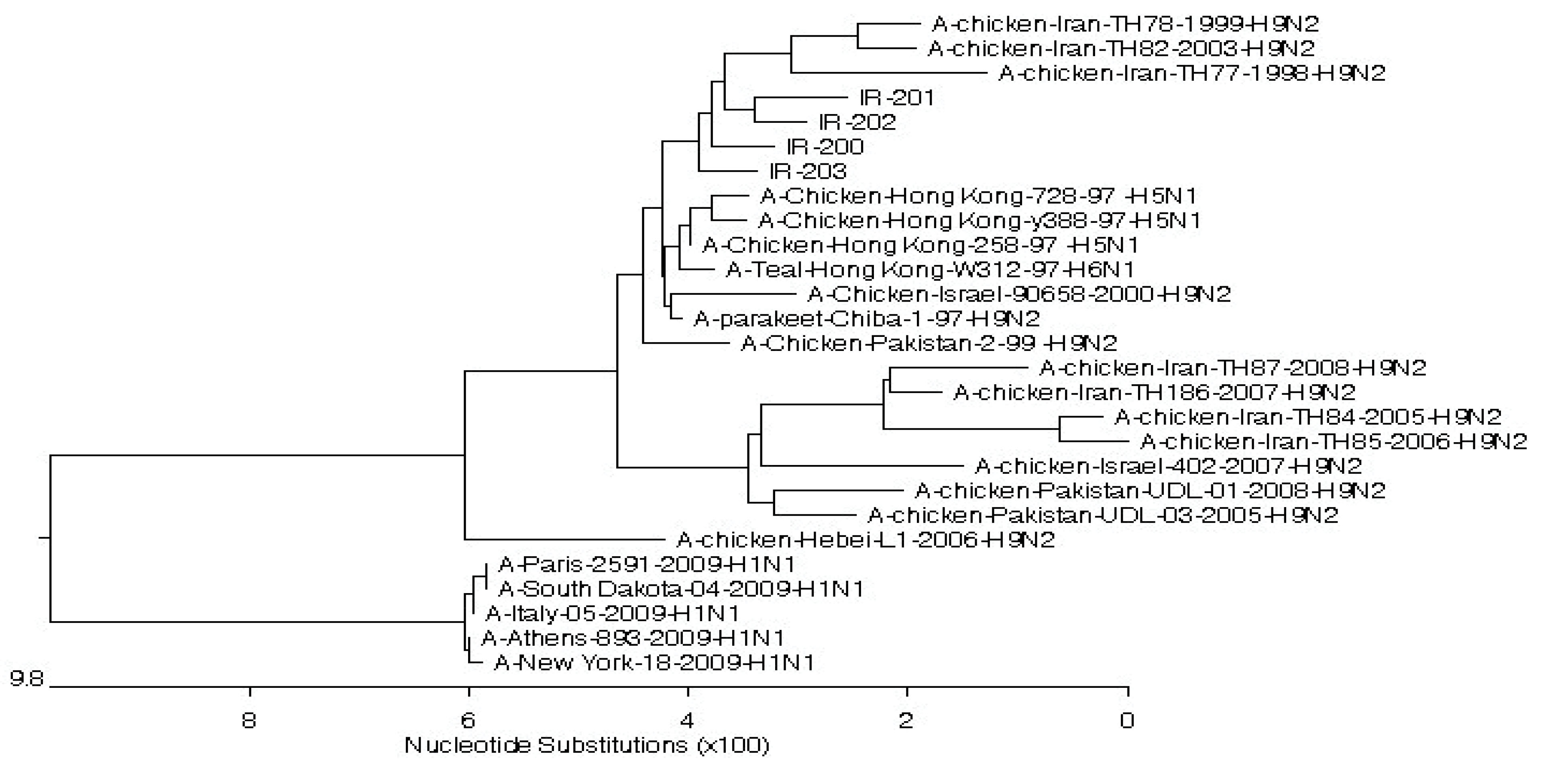
Figure 3.
The Phylogenic Tree of the Analyzed AIVs (IR-203, IR-205, IR-208 and IR-209) Compared to the Strains Registered in World Gene Bank.
.
The Phylogenic Tree of the Analyzed AIVs (IR-203, IR-205, IR-208 and IR-209) Compared to the Strains Registered in World Gene Bank.
Discussion
The analysis of nucleotide and amino acid sequences of the M gene in the H9N2 strains of the recent study revealed a high genomic relationship between these sequences and other previous Iranian H9N2 strains detected in recent decades. This homology is in any case above 90%. In their study, Ebrahimi et al reported the homology of the complete sequencing related to the M2 strain of Iranian H9N2 strains with other H9 and H5 strains obtained from different hosts and geographic regions between 92% and 98%. This similarity has been reported at the amino acid sequencing level between 97% and 100% (10). However, this high affinity represents the common source of H9N2 strains in Iran although a slight discrepancy between the strains of H9N2 in Iran leads to the formation of subcategories that cause the distribution of Iranian H9N2 strains among the other strains of H9N2 in the phylogenetic tree. These minor differences indicate that segment 7 of the H9N2 virus does not remain constant over time and may develop gradually. Further, branch lengths show the number of mutations that have occurred in the evolutionary time between the lineages. According to the report on the consolidation of segment 7 of the human influenza virus (11), in the process of an antigen shift from the H1N1 to the H2N2 strain and then the emergence of H3N2 (12), it seems that the probability of changes in the segment 7 of AIV is greater with the passage of time compared to human influenza.
The investigation of epitope regions on the M1 protein represented that the homology related to the amino acid sequence of the M1 protein of recent strains in epitope regions (i.e., 1, 2, and 3 regions) is 96%-100%. This homology in the cleavage site, the ion channel gate, the Zn binding site, and the nuclear localization sequence is 100%, which is identical with other reference strains. Therefore, it seems that no changes have been made in the functional and biological characteristics of these viruses during the last decade.
The comparison of recent strains with other strains of H9N2 from different countries demonstrates that the M gene sequence of the Iranian H9N2 strains of AIV is most similar to the Japanese strain of H9N2 originating from Pakistan (A/parakeet/Chiba/1/97(H9N2)). The high affinity of Iranian H9N2 strains to this isolate from Pakistan can help discover the origin of H9N2 strains in Iran. Further, a previous study estimated Pakistan as the origin of the H9N2 strain of Iran (4).
Recent H9N2 strains, in addition to being extremely similar to previous Iranian strains, are highly similar to the Hong Kong H5N1 strain (A/chicken/Hong Kong/728/97 (H5N1)). Unlike other analyzed strains, most recent strains have 100% similarity to the Hong Kong strains of H5N1 in the epitope area of the M1 protein. The biological and functional role of these epitopes is not well known and the pathogenicity of influenza viruses is affected by various factors. Accordingly, recent viruses may not have high pathogenicity in humans although there is the possibility of this ability due to high mutations. According to the previous reports, the probability of the Iranian H9N2 viruses becoming a highly pathogenic virus with the ability to transfer to humans is not far behind given the sequence of hemagglutinin and neuraminidase glycoproteins on the occurrence of Iranian H9N2 AIVs in the Hong Kong human influenza group (13).
Amantadine is an antiviral agent that specifically inhibits influenza A virus replication. It is sufficiently proved that amantadine and amantadine-derived compounds can block the proton channel formed by the M2 protein and prevent the required pH changes for the virus uncoating process (5). Resistance to amantadine occurs through a point mutation in each of the amino acid positions 26, 27, 30, and 31 or 34 of the M2 protein (14). Among the above-mentioned situations, mutation at position 31 was reported more than other situations among resistant strains (15,16). The analysis of recent AIV strains showed that three out of four strains had S31N substitution (serine to asparagine). In the other important determinants of resistance to amantadine, there is no change in the amino acid sequence of the strains. Therefore, it seems that one of these four strains is sensitive to amantadine while the remaining ones are resistant. In a previous study, Aghahossein Fanni et al reported the presence of amantadine-resistance among AIVs detected in 2007-2009 while the strains detected in 1998, 1999, and 2006 did not have resistance alternatives (17). Similarly, Yavarian et al found an increase in amantadine resistance, due to S31N mutation in the M2 channel protein, among human influenza H3N2 strains circulating in Iran during 2005-2007 (18). Given that the number of samples for checking the resistance to amantadine is insufficient, judgments about this subject in the H9N2 viruses of Iran need further investigation.
Conclusions
Although minor differences are observed in the M gene sequence, there may be more differences in other genes in AIV genes, which requires investigating several genes in the virus simultaneously. However, minor variations in the M gene provide the conditions for using this gene in designing the primer and the production of recombinant vaccines. In general, it is suggested that human and AIVs originated from commercial chickens, migratory and aquatic birds, be used to analyze the M gene and to investigate amantadine resistance.
Conflict of Interests
The authors declared no conflict of interests.
Acknowledgement
We appreciate the research deputy of Islamic Azad University, Shahrekord Branch for providing a grant to achieving examinations.
Ethical Approval
All animals received human care in compliance with the Guide for Care and Use of Laboratory Animals published by the National Institutes of Health (NIH publication No. 85-23, revised 1985). The study was approved by the Institutional Animal Care and Use Committee of our veterinary school.
Authors’ Contribution
Conceived and designed the experiments: MGA. Performed the experiments: MHS and MGA. Analyzed the sequences: MKD and MGA. Wrote the paper: SA, MHS, MKD and MGA.
Funding/Support
This research was supported by a grant from the Islamic Azad University, Shahrekord Branch (Grant no. 95-58725)
References
- Suarez DL, Sims LD. Influenza. In: Swayne DE, Glisson JR, McDougald LR, Nolan LK, Suarez DL, Nair VL, eds. Disease of Poultry. 13th ed. Ames: Wiley-Blackwell; 2013. p. 181-219.
- Arab M, Gholami-Ahangaran M, Jafarian-Dehkordi M. The molecular study of co-incidence of avian influenza (H9N2 subtype) and metapneumovirus in broiler chickens with respiratory syndrome in Isfahan province. Iranian Journal of Vetrinary Clinical Sciences 2017; 10(2):3-11. [ Google Scholar]
- Toroghi R, Momayez R. Biological and molecular characterization of Avian influenza virus (H9N2) isolates from Iran. Acta Virol 2006; 50(3):163-8. [ Google Scholar]
- Bashashati M, Vasfi Marandi M, Sabouri F. Genetic diversity of early (1998) and recent (2010) avian influenza H9N2 virus strains isolated from poultry in Iran. Arch Virol 2013; 158(10):2089-100. doi: 10.1007/s00705-013-1699-2 [Crossref] [ Google Scholar]
- Pielak RM, Chou JJ. Influenza M2 proton channels. Biochim Biophys Acta 2011; 1808(2):522-9. doi: 10.1016/j.bbamem.2010.04.015 [Crossref] [ Google Scholar]
- Tang Y, Zaitseva F, Lamb RA, Pinto LH. The gate of the influenza virus M2 proton channel is formed by a single tryptophan residue. J Biol Chem 2002; 277(42):39880-6. doi: 10.1074/jbc.M206582200 [Crossref] [ Google Scholar]
- Hoffmann E, Stech J, Guan Y, Webster RG, Perez DR. Universal primer set for the full-length amplification of all influenza A viruses. Arch Virol 2001; 146(12):2275-89. doi: 10.1007/s007050170002 [Crossref] [ Google Scholar]
- Li YP, Bang DD, Handberg KJ, Jorgensen PH, Zhang MF. Evaluation of the suitability of six host genes as internal control in real-time RT-PCR assays in chicken embryo cell cultures infected with infectious bursal disease virus. Vet Microbiol 2005; 110(3-4):155-65. doi: 10.1016/j.vetmic.2005.06.014 [Crossref] [ Google Scholar]
- Gholami-Ahangaran M, Shoushtari AH, Charkhkar S. Phylogenetic analysis of S1 protein in infectious bronchitis viruses that identified in broiler chickens in Isfahan. In: Symposium of Hygiene and Disease of Poultry. Shahrekord: Veterinary Faculty; 2008. p. 530-4. [Persian].
- Ebrahimi SM, Aghaiypour K, Nili H. Sequence analysis of M2 gene of avian influenza virus strain (A/Chicken/Iran/101/98 (H9N2)) as an oil vaccine seed. Iran J Biotechnol 2008; 6(4):235-8. [ Google Scholar]
- Hall RM, Air GM. Variation in nucleotide sequences coding for the N-terminal regions of the matrix and nonstructural proteins of influenza A viruses. J Virol 1981; 38(1):1-7. doi: 10.1128/jvi.38.1.1-7.1981 [Crossref] [ Google Scholar]
- Lindstrom SE, Cox NJ, Klimov A. Genetic analysis of human H2N2 and early H3N2 influenza viruses, 1957-1972: evidence for genetic divergence and multiple reassortment events. Virology 2004; 328(1):101-19. doi: 10.1016/j.virol.2004.06.009 [Crossref] [ Google Scholar]
- Ghorbani A, Moosakhani F, Mardani MV. Phylogenetic analysis of the hemagglutinin gene of recent H9N2 avian influenza viruses isolated from broiler flocks in Iran. Veterinarski Arh 2016; 86(1):95-109. [ Google Scholar]
- Dong G, Peng C, Luo J, Wang C, Han L, Wu B. Adamantane-resistant influenza a viruses in the world (1902-2013): frequency and distribution of M2 gene mutations. PLoS One 2015; 10(3):e0119115. doi: 10.1371/journal.pone.0119115 [Crossref] [ Google Scholar]
- Hata M, Tsuzuki M, Goto Y, Kumagai N, Harada M, Hashimoto M. High frequency of amantadine-resistant influenza A (H3N2) viruses in the 2005-2006 season and rapid detection of amantadine-resistant influenza A (H3N2) viruses by MAMA-PCR. Jpn J Infect Dis 2007; 60(4):202-4. [ Google Scholar]
- Townsend MB, Smagala JA, Dawson ED, Deyde V, Gubareva L, Klimov AI. Detection of adamantane-resistant influenza on a microarray. J Clin Virol 2008; 42(2):117-23. doi: 10.1016/j.jcv.2007.12.019 [Crossref] [ Google Scholar]
- Aghahossein Fanni A, Barin A, Moosakhani F, Ghalyanchi Langeroudi A. Genetical evaluation of resistance to amantadine in (H9N2) avian influenza virus in Iran between 1998 to 2009. Veterinary Clinical Pathology 2011; 5(4):1387-1395. [ Google Scholar]
- Yavarian J, Azad TM, Zheng X, Gregory V, Lin YP, Hay A. Amantadine resistance in relation to the evolution of influenza A(H3N2) viruses in Iran. Antiviral Res 2010; 88(2):193-6. doi: 10.1016/j.antiviral.2010.08.013 [Crossref] [ Google Scholar]