Avicenna Journal of Clinical Microbiology and Infection. 7(3):65-71.
doi: 10.34172/ajcmi.2020.15
Original Article
Re-construction of Co-expression Network of Genes Involved in Bacterial Cell Wall Synthesis and Their Role in Penicillin Resistance
Yasoub Shiri 1, *  , Amir Khodavirdipour 2, 3
, Amir Khodavirdipour 2, 3  , Nooshin Kalkali 4
, Nooshin Kalkali 4
Author information:
1Assistant Professor at Department of Agronomy and Plant Breeding, Agricultural Research Institute, University of Zabol, Iran.
2Division of Human Genetics, Department of Anatomy, St. John’s Hospital, Bangalore, India.
3Department of Biology, Faculty of Natural Sciences, University of Tabriz, Tabriz, Iran.
4Department of Biotechnology, Faculty of Agriculture, University of Zabol, Iran.
Abstract
Background: Peptidoglycan (Murein), which consists of disaccharide and amino acid chain subunits, has a key role in bacterial survival and ranks first in the line defense system against drug therapy. In addition, the transpeptidase enzyme plays an important role in cross-linking in bacterial cell walls. In Escherichia coli bacteria, cross-linking happens by proteins that have a D-D transpeptidase role and bond two amino acids of D-alanine together. These proteins are characterized by their affinity for and binding of penicillin thus they are called penicillin-binding proteins (PBPs). It should be noted that this bonding formation is prevented by the beta-lactam family as they have a similar structure to the above-mentioned proteins. The product of the idtD gene by characteristics such as L-D transpeptidase can catalyze the peptidoglycan structure in the bacterial cell wall in the presence of beta-lactam antibiotics.
Methods: In this study, around 426 interactions were identified between genes and approximately 20 genes with a key role in the process of bacterial cell wall synthesis by the reconstruction of 44 genes involved in bacterial cell wall synthesis.
Results: The idtD gene locus at the reconstructed network clearly shows that its catalytic activity is the side activity, and there won’t be a lag or disturbance in the procedure cell wall synthesis by removing it from the cycle. However, this side process causes the strengthening of the bacterial cell wall synthesis process against disorders arising by the presence of beta-lactam antibiotics.
Conclusions: These five genes in E. coli that furnish L-D transpeptidase properties include IdtA, IdtC, IdtD, IdtE, and mrdA out of which, IdtD is the most important gene in this process.
Keywords: Antimicrobial resistance, Cell wall, E.coli, Gene network, Murein, Penicillin
Copyright and License Information
© 2020 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Introduction
The cell wall plays a key role in bacterial survival and is considered as the first line of defense against drug therapies. In addition, gram-positive and Gram-negative bacteria have peptidoglycan structures in their cell walls which consist of disaccharide subunit repeat binding with cross-linked amino acids (1). Glycan strands are polymerized by the glycosyltransferase enzyme. Further, the transpeptidase enzyme has the main role in the creation and development of cross-linking in the bacterial cell wall. In the Escherichia coli, crossed bonds are controlled by proteins that have D-D transpeptidase properties and connect 2 D-alanine amino acids. Furthermore, these proteins have the ability to bind to penicillin, which is the reason they are called penicillin-binding proteins (PBPs). Moreover, these bonds are prevented by the beta-lactam family of antibiotics as they have similar structures to the above-mentioned bonds. The penicillin family of antibiotics has a single ring of beta-lactam in its structure thus it is also known as the beta-lactam antibiotic (2). Beta-lactam ring in the beta-lactam family of antibiotics and their similarity to the D-alanine/D-alanine structure can be observed in Figure 1. The similarity between the beta-lactam families of antibiotics and D-alanine/D-alanine in the cell wall prohibits the progress and function of the cell wall, and finally, causes cell death.
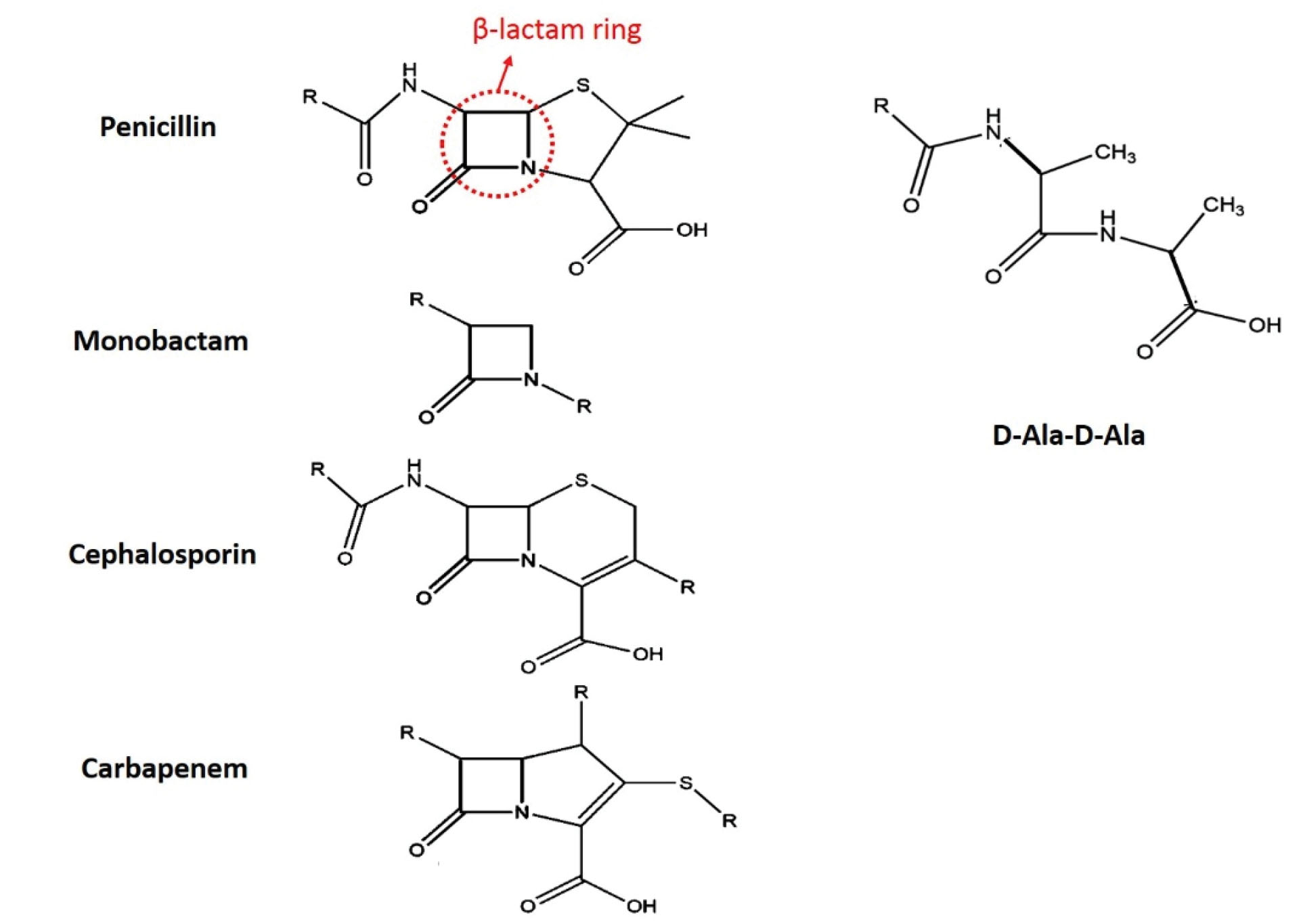
Figure 1.
Beta-lactam Ring in the Beta-lactam Families of Antibiotics (penicillin) and Their Similarities to D-alanine/D-alanine Bindings.
.
Beta-lactam Ring in the Beta-lactam Families of Antibiotics (penicillin) and Their Similarities to D-alanine/D-alanine Bindings.
Unusual interconnections that connect the remaining of two diaminopropionic acids were identified in early 1969 in E. coli, but the primary enzyme which is responsible for their formation remained unknown until later decades (3). These crossed bonds of DAP3 → DAP3consists of 3 and 10% of available interconnections in the extracted peptidoglycan from bacterial in exponential and stationary growth phases, respectively (3). Recently, it has been elucidated that the L,D transpeptidase (Idt) enzyme is responsible for this unusual interconnection and, unlike the common belief, these proteins are not from the PBPs (4). Previous research on the E. coli chromosome showed that it has five genes that are responsible for the L,D transpeptidase. Among these genes, namely, YcbB and YnhG can catalyze DAP3→DAP3 (5) and the other three genes (i.e., YcfS, ErfK, andYbiS) shall connect lipoproteins to peptidoglycans (6). Hugonnet et al demonstrated that YcbB encoded for a protein has the ability to function as a substitute for the D, D transpeptidase of the PBP family of proteins and thus causes the resistance to the beta-lactam family of antibiotics (7). Resistance to penicillin and other antibiotics from the beta-lactam family can be found in a wide range of gram-negative and gram-positive bacteria. Accordingly, the identification and study of genes involved in the resistance process can be a great help to overcome this problem and break the defense mechanism of bacteria against the beta-lactam family of antibiotics. To nail this aim using data deposited to databases and bioinformatics tools, the present study evaluated the co-expression network of genes involved in the synthesis of the bacterial cell wall and its role in antibacterial resistance.
Materials and Methods
In this study, published papers and findings and deposited data on UniProt were collected with regard to genes that were involved in the cell wall synthesis of bacteria (8), the details of which are presented in Table 1. Then, these genes were entered in the STRING database (9) and the network was expended three times. Finally, the network was obtained by 44 nodes with 426 edges. Additionally, other genes associated with this process were identified using parameters like co-expression and experiments. The obtained data from STRING analysis for the re-creation of the co-expression network purpose were uploaded into Cytoscape software (10). In addition, network topology was studied based on betweenness and closeness centrality parameters by Network Analyser software (11). An entity centrality of a complex network is named by betweenness centrality. Likewise, it is calculated based on all shortest edge pairs in a network. On the other hand, the closeness centrality is defined by the shortest distance mean from one node to all other nodes. In a simpler word, the higher amount of betweenness and closeness centrality for a node represents the importance of that node for the network (12,13). The ontology analysis of genes in the above network was performed by web-based software DAVID (14). Then, Benjamin P value was calculated for every single gene (15).
Table 1.
The Initial Genes Involved in Peptidoglycan Anabolism Which Entered Into the STRING Database
|
Gene Name
|
Gene ID
|
Definition
|
Reference |
|
mtgA
|
947728 |
Biosynthetic peptidoglycan transglycosylase |
PubMed: 18165305 |
|
ftsW
|
946322 |
Probable peptidoglycan glycosyltransferase |
PubMed: 11807049 |
|
mrcB
|
944843 |
Penicillin-binding protein 1B |
PubMed: 19458048 |
|
ftsI
|
944799 |
Peptidoglycan D,D-transpeptidase |
PubMed:9282742 |
|
mrcA
|
947907 |
Penicillin-binding protein 1A |
PubMed: 7006606 |
|
murA
|
947703 |
UDP-N-acetylglucosamine 1-carboxyvinyltransferase |
PubMed: 20392080 |
|
murE
|
944791 |
UDP-N-acetylmuramoyl-L-alanyl-D-glutamate--2,6-diaminopimelate ligase |
Pubmed: 11124264 |
|
dacB
|
947693 |
D-alanyl-D-alanine carboxypeptidase DacB |
PubMed: 2046551 |
|
mrdB
|
945238 |
Peptidoglycan glycosyltransferase |
PubMed: 27643381 |
|
pbpC
|
947152 |
Penicillin-binding protein 1C |
PubMed: 10542235 |
|
dacB
|
947693 |
D-alanyl-D-alanine carboxypeptidase |
PubMed: 2046551 |
|
dacC
|
945455 |
D-alanyl-D-alanine carboxypeptidase |
PubMed: 2046551 |
|
ldtD
|
945541 |
L,D-transpeptidase |
PubMed: 32486329 |
|
ycbB
|
945541 |
Probable L,D-transpeptidase |
PubMed: 18456808 |
|
mrdA
|
945240 |
Peptidoglycan D,D-transpeptidase |
PubMed: 20392080 |
Note. UDP-N: Uridine diphosphate N-acetylglucosamine.
Results and Discussion
The mechanism of antimicrobial-resistance to beta-lactam antibiotics is evaluated in 3 different ways. First, the mechanism is related to gram-negative bacteria that block the entry of antibiotics into the cell by changes in the structure of purines (pores in the bacterial cell wall). The second mechanism linked to the antimicrobial-resistant is common between gram-positive and -negative bacteria. These antibiotic-resistant bacteria have the capacity to breakdown the C-N bond in the beta-lactam ring. Finally, the third mechanism in antimicrobial-resistant bacteria to the beta-lactam family, the bacteria change the transpeptidase enzyme structure in such a way that no antibiotic can enter the bacteria. The second and third mechanisms were already reported in different strains of E. coli (16).
In general, 44 genes with a role in peptidoglycan synthesis in E. coli were selected based on our data mining and evaluation and analysis on the UniProt database (Table 2). Further, the interactions between the genes were identified using the STRING database. Furthermore, the centrality of the nodes in the protein-protein interaction network was calculated by applying the Network Analyzer tool, and finally, the co-expression network of the above genes was re-created by Cytoscape software. As shown in Figure 2, a complex network of genes was involved in the synthesis of the peptidoglycan process. The topology analysis elucidated that the murF gene by 23 connections had the highest connections with other genes in the network and then murA by 22 connections and murD, murE, murI, and ftsW genes each with 20 connections. The murA gene, along with mrcA, mrdB, mrdA, dacB, and ftsW had 0.020, 0.018, 0.0137, 0.0133, 0.0099, and 0.0092 betweenness centrality, respectively. The closeness centrality of each gene was an amount between 0 and 1. Further, 13 genes had the highest amount of closeness centrality equal to 1, including IdtD, murA, murC, murD, murE, murF, and murG.
Table 2.
Some Hub nodes in
Figure 2 Networks and the Metrical Information of Networks
|
Hub Nodes
|
Edge Count
|
Closeness Centrality
|
Betweenness Centrality
|
|
ldtD
|
3 |
1 |
0.00166113 |
|
pbpG
|
6 |
0.5 |
0.00221484 |
|
mepM
|
7 |
0.51851852 |
0.00415282 |
|
murJ
|
7 |
0.66666667 |
0.00198413 |
|
bacA
|
8 |
0.6 |
0.00913621 |
|
lpoB
|
8 |
0.58333333 |
0.00510863 |
|
alr
|
9 |
0.47692308 |
0.00909007 |
|
nagZ
|
10 |
0.5 |
0.00478564 |
|
dacB
|
11 |
0.60465116 |
0.00996678 |
|
mrcB
|
12 |
0.75 |
0.00361889 |
|
glmU
|
12 |
0.66666667 |
0.00293994 |
|
mrdA
|
15 |
0.78571429 |
0.01339187 |
|
mrcA
|
16 |
0.625 |
0.01889535 |
|
mrdB
|
17 |
1 |
0.01376101 |
|
murC
|
17 |
1 |
0.00277053 |
|
murG
|
19 |
1 |
0.00373359 |
|
ftsW
|
20 |
1 |
0.0092608 |
|
murD
|
20 |
1 |
0.00238161 |
|
murA
|
22 |
1 |
0.02042003 |
|
murF
|
23 |
1 |
0.00279689 |
| Total Number of Nodes |
Total Count of Edge |
Closeness Centrality Average |
Betweenness Centrality Average |
| 44 |
426 |
0.645646082 |
0.003347428 |
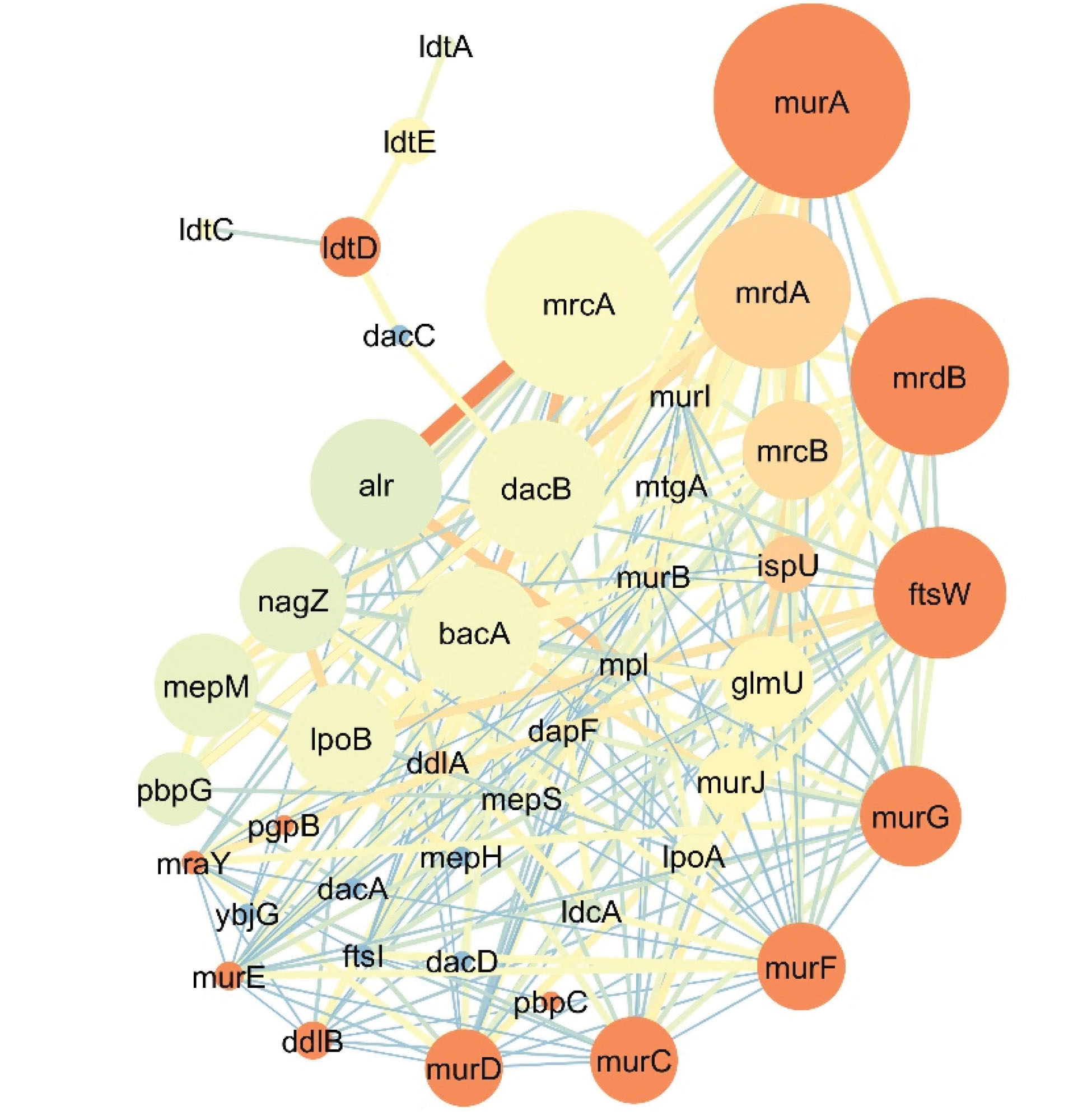
Figure 2.
Genes Involved in Peptidoglycan Wall Synthesis in E. coli.
Note. Node size depends on betweenness centrality (lower size for a lesser amount) and the color of the node varies from blue (the least closeness centrality) to red (highest closeness centrality). The color and thickness of each line (the connection between to genes) also vary from red and thick for a higher amount and blue and thin lines for fewer amounts.
.
Genes Involved in Peptidoglycan Wall Synthesis in E. coli.
Note. Node size depends on betweenness centrality (lower size for a lesser amount) and the color of the node varies from blue (the least closeness centrality) to red (highest closeness centrality). The color and thickness of each line (the connection between to genes) also vary from red and thick for a higher amount and blue and thin lines for fewer amounts.
The product of the murA gene is an enzyme named 1-carboxy vinyl transferase which catalyzes the cornerstone of bacterial cell wall biosynthesis (17). As shown in Figure 3, this enzyme catalyzes the biosynthesis in the presence of phosphoenolpyruvate and the uridine diphosphate N-acetylglucosamine molecule yield (1-carboxy vinyl)-α-D-glucosamine. A free phosphate is also a byproduct of this reaction (18). The suppression of the murA gene by fosfomycin antibiotics prevents the cell wall synthesis and subsequently bacterial death (18). More precisely, the re-created network and the topology analysis results elucidate that the murA gene has a key role in the process. The other genes from the Mur gene family (e.g., murB, murC, and the like) all play a role in cell wall synthesis. Eventually, the gene murG catalyzes the last reaction of the process, and subsequently, causes the formation of disaccharide-pent peptide production which is a subunit of the peptidoglycan cell wall (19). Moreover, the products of mrdA and mrdB catalyze the crossed binding of peptidoglycan cell walls and have a role in the width synthesis of the peptidoglycan cell wall, and finally, preserve and protect the bacterial structure and firmness (20). Most of the genes in this network are sensitive to the beta-lactam family of antibiotics (21). As mentioned earlier, each gene has connections with a higher number of other genes in the network. For example, the IdtDgene via dacC and dacB genes is connected to the main network. Although the dacB gene does not have a direct role in transpeptidation, it exclusively catalyzes DD-carboxy-peptidase and DD-endopeptidase reactions (22). On the other hand, dacC discards the remaining of the C-terminal of D-alanine from the precursors of peptide-sugar cell walls.
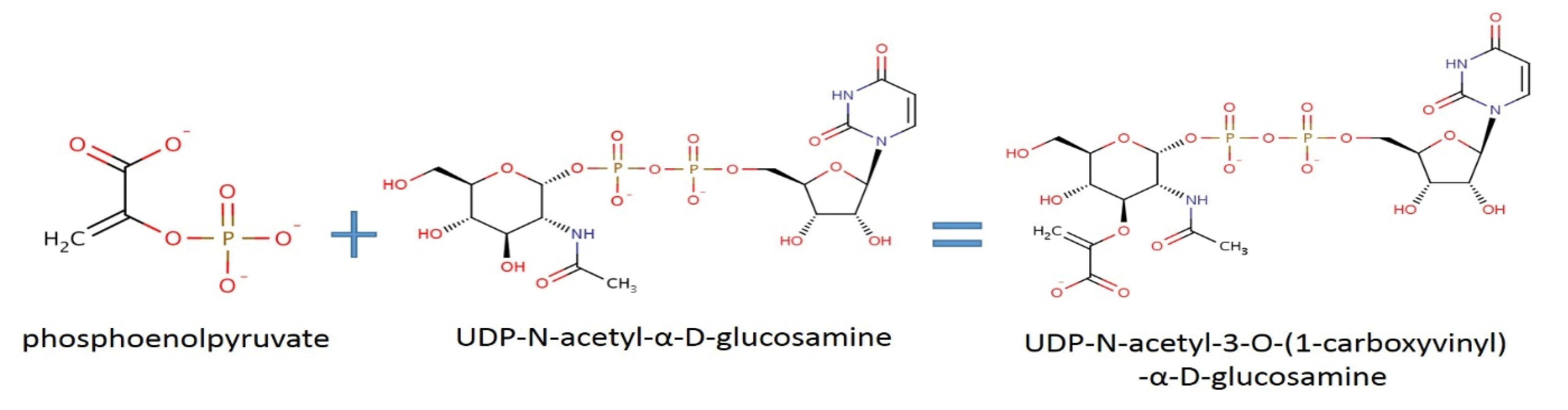
Figure 3.
Catalyzed Reaction of Carboxy Vinyl Transferase. Note. This reaction is the first step in the synthesis of bacterial cell walls.
.
Catalyzed Reaction of Carboxy Vinyl Transferase. Note. This reaction is the first step in the synthesis of bacterial cell walls.
In E. coli bacteria, the product of IdtD AKA YcbB has the ability to catalyze the reciprocal bindings of the amino acids in the peptidoglycan structure of the bacterial cell wall in the presence of the beta-lactam family of antibiotics since the catalyzed peptidyl binding is not D-D but is of L-D type (5). Despite the activity of IdtDin the presence of the beta-lactam family of antibiotics, it remains sensitive to the carbapenem, meropenem, and imipenem family of antibiotics. Additionally, it has been determined that copper can repress the activity of IdtG(7). The I dtD gene locus in the re-created network clearly demonstrates that the catalyzed reaction is a collateral process and there will be no disruption in the process of bacterial cell wall synthesis by removing this reaction. Conversely, this collateral process has a key role in bacterial resistance during the synthesis of the cell wall against the beta-lactam family of antibiotics. For a more detailed analysis of the role of the IdtD gene in E. coli, its exclusive co-expression network has been re-created, which is shown in Figure 4.
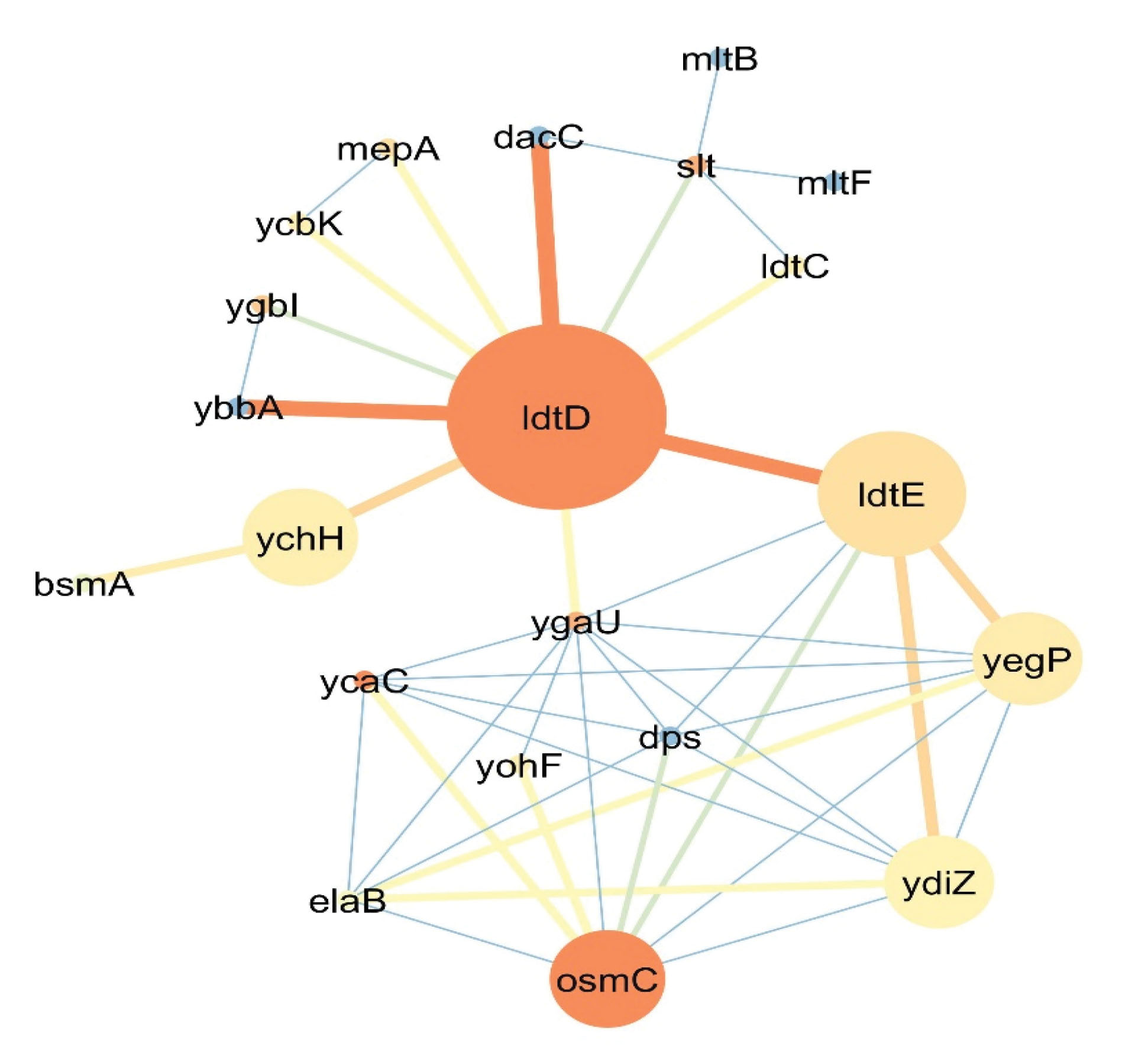
Figure 4.
Co-expression Network of the IdtD Gene that Is Responsible for D-L Transpeptidase Reciprocal Binding in E. coli Bacteria. Note. Node size depends on betweenness centrality (lower size for a lesser amount) and the color of the node differs from blue (the least closeness centrality) to red (highest closeness centrality). The color and thickness of each line (the connection between to genes) also vary from red and thick for a higher amount and blue and thin lines for fewer amounts.
.
Co-expression Network of the IdtD Gene that Is Responsible for D-L Transpeptidase Reciprocal Binding in E. coli Bacteria. Note. Node size depends on betweenness centrality (lower size for a lesser amount) and the color of the node differs from blue (the least closeness centrality) to red (highest closeness centrality). The color and thickness of each line (the connection between to genes) also vary from red and thick for a higher amount and blue and thin lines for fewer amounts.
Based on the data in Figure 4, co-expression network topology analysis shows that the IdtD gene has the most connections (n=10) in the network and other genes like ygaU, osmC, dps, and ydiZ by 9, 8, 7, and 7 connections are placed 2 to 5 in the ranking, respectively. Additionally, ycaC, osmC, and IdtD by the value of 1 have the highest closeness centrality and, from the betweenness centrality viewpoint of genes like IdtD (0.0578), IdtE (0.0236), and ychH (0.0078) have the highest value.
Based on the results in Figure 4, the IdtD gene had an important role in the L,D transpeptidase binding process. As previously mentioned, this gene catalyzes the DAP3 → DAP3. Another gene named IdtE AKA YnhG has the same function as the IdtG gene. According to a previous report, the upregulation of this gene in E. coli bacteria subsequently results in an increase in the DAP3 → DAP3binding in the bacterial cell wall structure (5). In defense against oxidative stress from exposure to the organic hydro peroxidase, osmC gene plays an osmotic induction role and preferably metabolizes organic hydro peroxidases more than inorganic hydrogen peroxidase (23). The co-expression of this gene by the upregulation of the D-L transpeptidase process shall clarify that D-L transpeptidase is activated in unfavorable environmental conditions and substitutes with the D,D transpeptidase function. Nonetheless, it also has an activity of 3 to 10% in favorable environmental conditions.
The box plot (Figure 5) displays the ontology analysis of genes in the co-expression network of bacterial cell wall synthesis. Out of 44 identified genes by the DAVID database based on molecular function, penicillin-binding with a P value of 7.2E-14 and 9 connections had the highest molecular function among all genes involved in the bacterial cell wall synthesis process. This finding proved that bacterial cell wall synthesis is extremely under the influence of the beta-lactam family of antibiotics. Binding capability to the magnesium(Mg) ion by 6 genes and the P value of 8.2E-2 is one of the active molecular functions among bacterial cell wall synthesis. These genes include D-alanine ligase A (ddlA), D-alanine ligase B (ddlB), L-alanine ligase (murC), meso-diaminopimelate ligase (murE), glucosamine 1-phosphate acetyltransferase (glmU), and finally, undecaprenyl-pyrophosphate syntheses (ispU). By considering the obtained data, the Mg enzyme was the co-factor of these enzymes and had a key role in bacterial cell wall synthesis. In addition, five genes of IdtA, IdtC, IdtD, IdtE, and mrdA had the D-L transpeptidase function and were observed in the co-expression network (Figure 4). The IdtD gene is the most key gene and responsible for D-L transpeptidase functionality in E. coli bacteria. From the locus point of view, these genes were evaluated and the results showed that all the above-mentioned genes are located in a membrane or the cell wall itself. From the biopathway perspective, the cell shape regulatory and peptidoglycan biosynthesis pathways with 39 (P value of 4.6E-73) and 38 (P value of 1.2E-62) genes are the two most active biopathways, respectively. It should be noted that each gene can present in different pathways with numerous different functions.
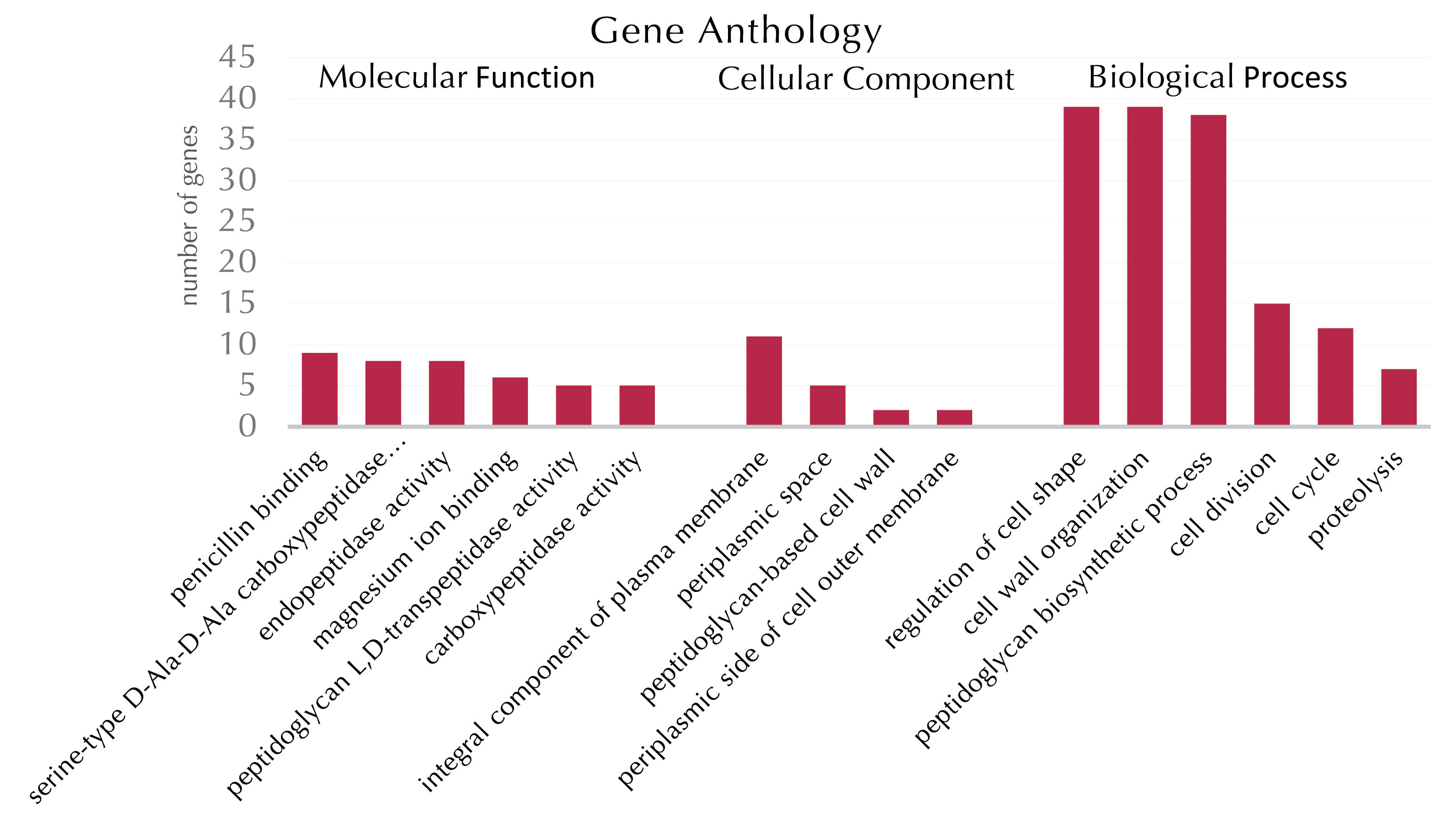
Figure 5.
Ontology Analysis Chart of Genes Involved in Bacterial Cell Wall Synthesis Process
.
Ontology Analysis Chart of Genes Involved in Bacterial Cell Wall Synthesis Process
Conclusions
In this study, about 426 interactions were identified between genes and approximately 20 genes with a key role in the process of bacterial cell wall synthesis by the reconstruction of 44 genes involved in bacterial cell wall synthesis. E. coli bacteria has the resistance machinery and mechanism to the penicillin (beta-lactam) family of anti-biotic. This process can proceed via different pathways. However, the cell wall structure changes, and the alteration and prevention of beta-lactam antibiotic efficacy in the bacterial cell wall synthesis process is one of the main mechanisms in bacterial resistance. In E. coli bacteria, the product of the IdtD gene has the ability to catalyze the reciprocal bindings of amino acids in the presence of the beta-lactam family of antibiotics. In addition, the IdtD gene locus in the re-created network clearly suggests that catalyzing activity is a collateral process, and there will be no disruption to the bacterial cell wall synthesis by removing it from the network. Nevertheless, this collateral process causes strengthening the synthesis pathway against disruption by the presence of the beta-lactam family of antibiotics. It seems that the D-L transpeptidase mechanism is activated in unfavorable environmental conditions, replacing the D, D transpeptidase mechanism. However, the amount of activity goes up to 10% in favorable environmental conditions. Further, the magnesium co-factor has a key role in bacterial cell wall synthesis. Overall, 5 genes in E. coli have D-L transpeptidase function, including IdtA, IdtC, IdtD, IdtE,andmrdA. Finally, theIdtD gene has an essential role in the D-L transpeptidase function in E. coli.
Conflict of Interests
The authors confirm that there is no conflict of interests.
Acknowledgment
We would like to thank the University of Zabol for its full support and financial aids (Grant number: 9618-127). Additionally, we would like to thank all colleagues and administrative staff of the research division of the University of Zabol.
References
- Typas A, Banzhaf M, Gross CA, Vollmer W. From the regulation of peptidoglycan synthesis to bacterial growth and morphology. Nat Rev Microbiol 2011; 10(2):123-36. doi: 10.1038/nrmicro2677 [Crossref] [ Google Scholar]
- Kong KF, Schneper L, Mathee K. Beta-lactam antibiotics: from antibiosis to resistance and bacteriology. APMIS 2010; 118(1):1-36. doi: 10.1111/j.1600-0463.2009.02563.x [Crossref] [ Google Scholar]
- Schwarz U, Asmus A, Frank H. Autolytic enzymes and cell division of Escherichia coli. J Mol Biol 1969; 41(3):419-29. doi: 10.1016/0022-2836(69)90285-x [Crossref] [ Google Scholar]
- Mainardi JL, Villet R, Bugg TD, Mayer C, Arthur M. Evolution of peptidoglycan biosynthesis under the selective pressure of antibiotics in gram-positive bacteria. FEMS Microbiol Rev 2008; 32(2):386-408. doi: 10.1111/j.1574-6976.2007.00097.x [Crossref] [ Google Scholar]
- Magnet S, Dubost L, Marie A, Arthur M, Gutmann L. Identification of the L, D-transpeptidases for peptidoglycan cross-linking in Escherichia coli. J Bacteriol 2008; 190(13):4782-5. doi: 10.1128/jb.00025-08 [Crossref] [ Google Scholar]
- Magnet S, Bellais S, Dubost L, Fourgeaud M, Mainardi JL, Petit-Frère S. Identification of the L, D-transpeptidases responsible for attachment of the Braun lipoprotein to Escherichia coli peptidoglycan. J Bacteriol 2007; 189(10):3927-31. doi: 10.1128/jb.00084-07 [Crossref] [ Google Scholar]
- Hugonnet JE, Mengin-Lecreulx D, Monton A, den Blaauwen T, Carbonnelle E, Veckerlé C. Factors essential for L, D-transpeptidase-mediated peptidoglycan cross-linking and β-lactam resistance in Escherichia coli. Elife 2016; 5. doi: 10.7554/eLife.19469 [Crossref]
- Apweiler R, Bairoch A, Wu CH, Barker WC, Boeckmann B, Ferro S. UniProt: the Universal Protein knowledgebase. Nucleic Acids Res 2004; 32:D115-9. doi: 10.1093/nar/gkh131 [Crossref] [ Google Scholar]
- Szklarczyk D, Morris JH, Cook H, Kuhn M, Wyder S, Simonovic M. The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res 2017; 45(D1):D362-D8. doi: 10.1093/nar/gkw937 [Crossref] [ Google Scholar]
- Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 2003; 13(11):2498-504. doi: 10.1101/gr.1239303 [Crossref] [ Google Scholar]
- Assenov Y, Ramírez F, Schelhorn SE, Lengauer T, Albrecht M. Computing topological parameters of biological networks. Bioinformatics 2008; 24(2):282-4. doi: 10.1093/bioinformatics/btm554 [Crossref] [ Google Scholar]
- Shiri Y, Solouki M, Ebrahimie E, Emamjomeh A, Zahiri J. Gibberellin causes wide transcriptional modifications in the early stage of grape cluster development. Genomics 2020; 112(1):820-30. doi: 10.1016/j.ygeno.2019.05.022 [Crossref] [ Google Scholar]
- Shiri Y, Solouki M, Ebrahimie E, Emamjomeh A, Zahiri J. Unraveling the transcriptional complexity of compactness in Sistan grape cluster. Plant Sci 2018; 270:198-208. doi: 10.1016/j.plantsci.2018.02.011 [Crossref] [ Google Scholar]
- Jiao X, Sherman BT, Huang da W, Stephens R, Baseler MW, Lane HC. DAVID-WS: a stateful web service to facilitate gene/protein list analysis. Bioinformatics 2012; 28(13):1805-6. doi: 10.1093/bioinformatics/bts251 [Crossref] [ Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 1995; 57(1):289-300. [ Google Scholar]
- Shiri Y, Karimiyan MA. Reconstitution of gene network on penicillin resistance in E coli using databases information. New Findings in Veterinary Microbiology 2020; 2(2):1-9. doi: 10.35066/j040.2019.701.[Persian] [Crossref] [ Google Scholar]
- Zhu JY, Yang Y, Han H, Betzi S, Olesen SH, Marsilio F. Functional consequence of covalent reaction of phosphoenolpyruvate with UDP-N-acetylglucosamine 1-carboxyvinyltransferase (MurA). J Biol Chem 2012; 287(16):12657-67. doi: 10.1074/jbc.M112.342725 [Crossref] [ Google Scholar]
- Olesen SH, Ingles DJ, Yang Y, Schönbrunn E. Differential antibacterial properties of the MurA inhibitors terreic acid and fosfomycin. J Basic Microbiol 2014; 54(4):322-6. doi: 10.1002/jobm.201200617 [Crossref] [ Google Scholar]
- Bupp K, van Heijenoort J. The final step of peptidoglycan subunit assembly in Escherichia coli occurs in the cytoplasm. J Bacteriol 1993; 175(6):1841-3. doi: 10.1128/jb.175.6.1841-1843.1993 [Crossref] [ Google Scholar]
- Cho H, Wivagg CN, Kapoor M, Barry Z, Rohs PDA, Suh H. Bacterial cell wall biogenesis is mediated by SEDS and PBP polymerase families functioning semi-autonomously. Nat Microbiol 2016; 1:16172. doi: 10.1038/nmicrobiol.2016.172 [Crossref] [ Google Scholar]
- Den Blaauwen T, Aarsman ME, Vischer NO, Nanninga N. Penicillin-binding protein PBP2 of Escherichia coli localizes preferentially in the lateral wall and at mid-cell in comparison with the old cell pole. Mol Microbiol 2003; 47(2):539-47. doi: 10.1046/j.1365-2958.2003.03316.x [Crossref] [ Google Scholar]
- Korat B, Mottl H, Keck W. Penicillin-binding protein 4 of Escherichia coli: molecular cloning of the dacB gene, controlled overexpression, and alterations in murein composition. Mol Microbiol 1991; 5(3):675-84. doi: 10.1111/j.1365-2958.1991.tb00739.x [Crossref] [ Google Scholar]
- Lesniak J, Barton WA, Nikolov DB. Structural and functional features of the Escherichia coli hydroperoxide resistance protein OsmC. Protein Sci 2003; 12(12):2838-43. doi: 10.1110/ps.03375603 [Crossref] [ Google Scholar]