Molecular Identification of MefE and AmpC Resistance Genes in ATCC Bacteria
Avicenna J Clin Microbiol Infect, 6(4), 142-143; DOI:10.34172/ajcmi.2019.26
Letter
Molecular Identification of MefE and AmpC Resistance Genes in ATCC Bacteria
Omid Pouresmaeil1, Hengameh Zandi1, Davood Kalantar-Neyestanaki2, Sahel Safaei3, Mehdi Fatahi-Bafghi1*, Mahmood Vakili4
1
Department of Microbiology, Faculty of Medicine, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
2
Department of Microbiology, Faculty of Medicine, Kerman University of Medical Sciences, Kerman, Iran
3
International Campus, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
4
Health Monitoring Research Center, School of Medicine, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
*Corresponding author: Mehdi Fatahi-Bafghi, Department of Microbiology, Faculty of Medicine, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. Email address: mehdifatahi@ssu.ac.ir
Abstract
Dear Editor,
Enterococci are gram-positive bacteria and the source of recurrent nosocomial infections with high levels of antibiotic resistance (1), of which we can name resistance to cephalosporins, aminoglycosides, monobactams, penicillinase resistance penicillins, and most importantly, vancomycin (2). Between 85% to 90% of the enterococci infections are caused by Enterococcus faecium (2), and macrolide-lincosamide-streptogramin is an antibiotic, which can be useful for treating enterococcal infections (1). M phenotype refers to a resistance mechanism to macrolides (such as erythromycin) and includes active-drug efflux pumps that are encoded by mef genes (3,4). For the first time, bacterial antibiotic efflux was reported in 1970 (3). The presence of mef genes have been reported in previous studies (6). In the current study, we reported mefE gene in E. faecium ATCC (American Type Culture Collection) 51559. Acinetobacter baumannii is a gram-negative bacterium that has turned into a great concern in the health care centers especially in intensive care units (4). The name Acinetobacter is originated from akinetos, a Greek word meaning non-motile (4). A. baumannii is a member of “ESKAPE” (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, A. baumannii, Pseudomonas aeruginosa and Enterobacter spp.) group which consist of multi-drug resistant organisms (4). It is one of the important causes of nosocomial infections (5). Among these multi-drug resistance traits, chromosomal AmpC, a non-inducible cephalosporinase, was already reported in A. baumannii genome (4). In this study, we reported AmpC resistance gene in A. baumannii ATCC 19606. The mentioned ATCC bacteria, which were isolated from clinical samples for the first time (6,7), were bought commercially. The Clinical and Laboratory Standards Institute (CLSI) guidelines were followed for the determination of resistance phenotypes (8). Suspensions of both A. baumannii ATCC 19606 and E. faecium ATCC 51559 were prepared with an opacity equivalent to 0.5 McFarland solution, and subsequently cultured on separate Mueller-Hinton agar plates. An ampicillin (10 μg) disc was used to check the phenotypic resistance in A. baumannii ATCC 19606 and an erythromycin (15 μg) disc for E. faecium ATCC 51559. The plates were then incubated for 24 hours at 35˚C. Staphylococcus aureus ATCC 25923 was used for the quality control of both discs. Both A. baumannii ATCC 19606 and E. faecium ATCC 51559 exhibited resistance to the above-mentioned antibiotic discs as shown in Figure 1. In the next step, DNA was extracted using boiling method (9); a loopful of each bacterium was suspended in 1 mL of distilled water and boiled for 15 minutes. Then each microtube was centrifuged at 15 000 g for 10 minutes. The DNA containing supernatants were used for PCR. The primers F: 5’-CAATATGGGCAGGGCAAG-3’ and R: 5’-AAGCTGTTCCAATGCTACGG-3’ were utilized for MefE gene detection (10), and the primers F: 5’- TAAACACCACATATGTTCCG-3’ and R: 5’- ACTTACTTCAACTCGCGACG-3’ for the AmpC gene detection (11). Both PCRs were carried out using 10 μL of the Master Mix Red (Amplicon, Denmark), 0.5 μL of each primer, and 3 μL of the respective DNA templates, and then brought to a total volume of 25 μL by adding deionized distilled water. Reaction cycles for MefE gene included an initial 7-minute denaturation at 95˚C, followed by 44 cycles of: denaturation at 95˚C for 30 seconds, annealing at 65˚C for 30 seconds, and extension at 72˚C for 50 seconds, and final extension step at 72˚C for 5 minutes. In the case of AmpC gene, the initial denaturation was at 95˚C for 3 minutes, followed by 35 cycles of: 94˚C for 1 minute, 56˚C for 1 minute, 72˚C for 2 minutes, and a final extension at 72˚C for 10 minutes. A 1.5% agarose gel electrophoresis was used for the analysis of PCR products. The expected bands, 317 bp for MefE gene and 663 bp for AmpC gene, were observed (Figure 2). PCR products were sent to the Bioneer Company, South Korea, for sequencing. The results were confirmed by sequencing. Finally, sequences were registered on the GenBank. The accession numbers for A. baumannii and E. faecium are MF290419 and MF290421, respectively. Since the antibiotic resistance, especially multidrug resistance is a critical medical issue, detection and assessment of antibiotic resistance genes is of great importance.
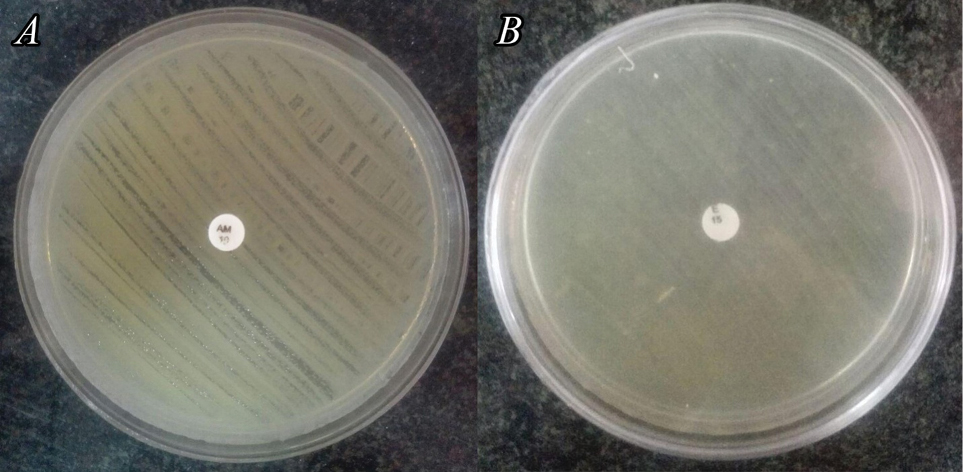
Detection of Phenotypic Resistance.
A: A. baumannii ATCC 19606 exhibits resistance phenotype to ampicillin (10 µg) disc.
B: E. faecium ATCC 51559 exhibits resistance phenotype to erythromycin (15 µg) disc.

Expected Bands on 1.5% Agarose Gel Electrophoresis. AmpC gene: 663 bp; MefE gene: 317 bp.
References
- Portillo A, Ruiz-Larrea F, Zarazaga M, Alonso A, Martinez JL, Torres C. Macrolide resistance genes in Enterococcus spp. Antimicrob Agents Chemother 2000;44(4):967-71. doi: 10.1128/aac.44.4.967-971.2000. [Crossref]
- Morse S, Carroll KC, Butel J. Jawetz Melnick & Adelbergs Medical Microbiology. 27th ed. McGraw-Hill Education; 2015.
- Poole K. Efflux pumps as antimicrobial resistance mechanisms. Ann Med 2007;39(3):162-76. doi: 10.1080/07853890701195262. [Crossref]
- Howard A, O’Donoghue M, Feeney A, Sleator RD. Acinetobacter baumannii: an emerging opportunistic pathogen. Virulence 2012;3(3):243-50. doi: 10.4161/viru.19700. [Crossref]
- Gerson S, Betts JW, Lucaßen K, Nodari CS, Wille J, Josten M, et al. Investigation of novel pmrB and eptA mutations in isogenic Acinetobacter baumannii isolates associated with colistin resistance and increased virulence in vivo. Antimicrob Agents Chemother 2019;63(3). doi: 10.1128/aac.01586-18. [Crossref]
- Landman D, Mobarakai NK, Quale JM. Novel antibiotic regimens against Enterococcus faecium resistant to ampicillin, vancomycin, and gentamicin. Antimicrob Agents Chemother 1993;37(9):1904-8. doi: 10.1128/aac.37.9.1904. [Crossref]
- Bouvet PJ, Grimont PA. Taxonomy of the genus Acinetobacter with the recognition of Acinetobacter baumannii sp. nov., Acinetobacter haemolyticus sp. nov., Acinetobacter johnsonii sp. nov., and Acinetobacter junii sp. nov. and emended descriptions of Acinetobacter calcoaceticus and Acinetobacter lwoffii. Int J Syst Evol Microbiol 1986;36(2):228-40. doi: 10.1099/00207713-36-2-228. [Crossref]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing. Wayne, PA: CLSI; 2016. p. 26.
- Cheng S, McCleskey FK, Gress MJ, Petroziello JM, Liu R, Namdari H, et al. A PCR assay for identification of Enterococcus faecium. J Clin Microbiol 1997;35(5):1248-50.
- Malhotra-Kumar S, Lammens C, Piessens J, Goossens H. Multiplex PCR for simultaneous detection of macrolide and tetracycline resistance determinants in streptococci. Antimicrob Agents Chemother 2005;49(11):4798-800. doi: 10.1128/aac.49.11.4798-4800.2005. [Crossref]
- Bou G, Martinez-Beltran J. Cloning, nucleotide sequencing, and analysis of the gene encoding an AmpC beta-lactamase in Acinetobacter baumannii. Antimicrob Agents Chemother 2000;44(2):428-32. doi: 10.1128/aac.44.2.428-432.2000. [Crossref]