Avicenna Journal of Clinical Microbiology and Infection. 6(3):88-94.
doi: 10.34172/ajcmi.2019.16
Research Article
Inhibition of TEMbla Producing Escherichia coli Isolated From Poultry Colibacillosis Using Cinnamomum camphora and Syzygium aromaticum Essential Oils
Bahar Malekpour 1  , Khatereh Kafshdouzan 2, *
, Khatereh Kafshdouzan 2, *  , Ashkan Jebelli Javan 3, Mohammad Reza Salimi Bejestani 2
, Ashkan Jebelli Javan 3, Mohammad Reza Salimi Bejestani 2
Author information:
1Student Research Committee, Faculty of Veterinary Medicine, Semnan University, Semnan, Iran
2Department of Pathobiology, Faculty of Veterinary Medicine, Semnan University, Semnan, Iran
3Department of Food Hygiene, Faculty of Veterinary Medicine, Semnan University, Semnan, Iran
*
Corresponding author: Khatereh Kafshdouzan, Department of Pathobiology, Faculty of Veterinary Medicine, University of Semnan, Semnan, Iran, Tel: +98-23-33654215, Fax: +98- 23-33654214 Email:
kafshdouzan@semnan.ac.ir
Abstract
Background: Antibiotic resistance transmission through the food chain is considered as a major health challenge. The combination of essential oils (EOs) with synergistic or additive effects regarding enhancing the antimicrobial activity is an applied approach for controlling foodborne pathogens and improving food safety. The aim of this study was to evaluate the combined effect of Cinnamomum camphora (cinnamon) and Syzygium aromaticum (clove) EOs against TEMbla produced by Escherichia coli isolated from poultry colibacillosis.
Methods: To this end, 100 E. coli isolated from the viscera of broilers suspected of colibacillosis, were examined to detect the ESBL produced by the combined disk method according to The Clinical and Laboratory Standards Institute (CLSI). In addition, TEMbla presence was detected by the polymerase chain reaction method. Finally, the antibacterial activity of cinnamon and clove EOs was studied against TEMbla harboring isolates alone and in combination with the broth microdilution method and fractional inhibitory concentration (FIC) index.
Results: Based on the results, 32/88 (36.3%) of the tested samples produced ESBL and 20/32 (62.5%) were found to harbor TEM. All the TEMbla produced by E. coli investigated by the broth microdilution assay were sensitive to cinnamon and clove EOs in the range of 400 to 1600 and 800 to 3200 ppm, respectively. FIC indices (ranging from 0.56 to 1) suggested their synergistic inhibitory effect on nine isolates and additive inhibitory effect against 11 isolates.
Conclusions: The results of this study indicated that the combination of cinnamon and clove can inhibit the growth of E. coli harboring the TEMbla gene and thus it can be suggested as a safe bio-preservative. However, further studies should be conducted to investigate the potential interaction between the EOs and food matrix components.
Keywords: Drug resistance, Microbial, Escherichia coli, Cinnamon camphora, Syzygium aromaticum, Food safety
Copyright and License Information
© 2019 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Background
Bacterial resistance to antimicrobial agents is a global important concern that has quickly developed worldwide. Among several diverse antimicrobial resistance mechanisms studied in bacteria, the production of extended-spectrum beta-lactamases (ESBLs) is one of the most important mechanisms which has emerged frequently (1). ESBLs are plasmid-encoded enzymes, produced by some bacteria such as Escherichia coli, and Klebsiella pneumoniae spp, which hydrolyse and inactivate a wide variety of beta-lactam antibiotics such as the penicillins. In addition, they are considered as the first, second, and third generations of cephalosporins and aztreonam. The TEM beta-lactamase (named after the patient Temoneira) is an enzyme that is frequently detected in E. coli and causes an infection in humans and food-producing animals (2).
Extraintestinal pathogenic E. coli (ExPEC), as a causative agent of avian colibacillosis, also causes some infectious diseases such as urinary tract infections, bacteremia, and meningitis in humans. Similar characteristics between the ExPEC in humans and poultry have led to the hypothesis of the zoonotic potential of poultry strains (3). Although the zoonotic risk to humans from chicken-source E. coli is not fully elucidated, recent studies have suggested that poultry products, particularly meat, can be a source of ExPEC strain transmission to humans (4). Several studies have increasingly reported the presence of the ESBL-producing E. coli in animal foods such as cattle and poultry in the last decade (2,5). Thus, contaminated chicken meat and/or meat products are considered as a potential swift rout for spreading this kind of contamination (6). The presence of similar or clonally related antibiotic-resistant bacteria with animal origin in the human population, who had no occupational exposure providing documents no proven consumption and/or handling of animal foods, play an important role in the transmission of antibiotic-resistant bacteria to humans (7,8).
In recent years, the contamination of poultry meat with the ESBL-producing E. coli is inevitable due to the over prevalence of infectious diseases such as colibacillosis in poultry and the widespread use of various antibiotics in this industry (2). The presence of E. coli possessing the antibiotic resistance genes in the chicken may cause carcass contamination during the slaughter. Meat contamination with antibiotic-resistant bacteria, along with developing foodborne diseases also promotes the spread of antibiotic resistance genes in the environment and is considered as a major potential risk for passing antibiotic resistance to the human being (2,3,8).
Although food processing and the use of chemical preservatives are commonly used to increase food shelf life, consumers demonstrate increasing concern regarding the use of synthetic chemical additives in foods and thus food industries seek to find natural alternatives in order to reduce the survival rate of microorganisms in food systems. The most applicable method in this regard is the use of essential oils (EOs) derived from medical plants (e.g., oregano, thyme, basil, clove, and cinnamon) or their constituents, individually or in combination, which is known as bio-preservative (9-11).
The EOs usually possess antimicrobial activity not only against bacteria but also against fungi, protozoans, and viruses, which is especially important in mixed infections. Furthermore, the EOs have antioxidant, immune-modulatory, and anti-inflammatory activities (12,13).
Syzygium aromaticumcommonly known as the clove belongs to the Myrtaceae family. The medicinal properties of the clove EO as an antiseptic and analgesic substance and its safety is well studied and approved by the U.S. Food and Drug Administration. Moreover, the antimicrobial activity of clove EOs is frequently investigated against many food-borne pathogens like Listeria monocytogenes, Campylobacter jejuni, Staphylococcus aureus, and E. coli (14-16).
Cinnamomum camphora, as a member of the Lauraceae family, is native to most countries in Southeast Asia and has been introduced to many other countries. According to several studies, eugenol and cinnamaldehyde as the main components of these EOs have an effective antimicrobial activity against both gram-positive and gram-negative bacteria (i.e., C. jejuni, Salmonella. spp, L. monocytogenes, S. aureus, Pseudomonas aeruginosa, and E. coli) (7,14,16).
Nowadays, the simultaneous application of the EOs or their components is a new approach for enhancing the efficacy of the EOs in foods because a large amount of EOs is needed to obtain an optimal antimicrobial result. In addition, those obtained in the in vitro assays can have adverse effects on food flavors (12). A combination of the EOs in addition to preventing unpleasant taste has potentially significant advantages such as reducing undesirable effects while increasing the stability or bioavailability of free agents, and obtaining an adequate antibacterial effect with relatively small doses (12,17).
The combination effects of two or more EOs are usually evaluated by the fractional inhibitory concentration (FIC) method, which is a measure to determine the interaction between two or more drugs intended to be used in the combination (18).
Similarly, the antibacterial activity effects of the EOs are typically studied on culture collection strains, whose antibiotic susceptibility profile is unclear. Therefore, limited knowledge is available about the antibacterial effects of the EOs on resistant bacterial strains. The transmission of antibiotic resistance agents through animal foods has become an important concern around the world. Accordingly, this study aimed to evaluate the antibacterial activity of cinnamon and clove EOs in different concentrations against TEMbla producing E. coli isolated from poultry colibacillosis and to determine their synergistic or antagonistic effects by the FIC index. To the best of our knowledge, this is the first study that investigated the combined effects of two common bio-preservatives against E. coli possessing TEMbla resistance gene.
Materials and Methods
Isolation of Escherichia coli
A total of 110 isolates were collected from poultry farms and veterinary laboratories in Semnan, Iran from November 2015 to April 2016. The samples were isolated aseptically from the heart, liver, and air sac lesions of broilers suspected to colibacillosis and identified as E. coli by standard biochemical tests. Further, the E. coli isolates were stocked in brain heart infusion (BHI) broth (Merk, Germany) with 15% glycerol at -20°C until further evaluation.
Extended-Spectrum Beta-Lactamase Detection by Combined Disk Method
All isolates were examined for antimicrobial susceptibility by Kirby-Bauer disk diffusion method on Mueller-Hinton agar (Merck, Germany) using overnight cultures at a 0.5 McFarland standard. Furthermore, antibiotic susceptibility was studied against chloramphenicol (30 µg), ampicillin (10 µg), cefalexin (30 µg), cefepime (30 µg), gentamicin (10 µg), tetracycline (30 µg), and ciprofloxacin (5 µg) that were provided from Padtan Teb, Iran. To confirm ESBL-producing isolates, resistant phenotypes related to the ESBLs were detected by performing the combined disk method (MAST® D67C). The disks were placed onto the inoculated medium with sufficient space between them, including ceftazidime 30 µg, ceftazidime 30/clavulanic acid 10 µg, cefotaxime 30 µg, cefotaxime 30/clavulanic acid 10 µg, cefpodoxime 10 µg, and cefpodoxime 10/clavulanic acid 1 µg. Then, the plates were incubated at 35°C for 16-18 hours, followed by measuring and recording any observed zone of inhibition. An increase in the zone diameter (≥5 mm) between the zone of an antibiotic disk and their respective antibiotic-clavulanate disk is confirmed as the ESBL-producing E. coli (19).The results were interpreted according to the Clinical and Laboratory Standards Institute (CLSI) guidelines 2017. E. coli ATCC 25922, was used as a quality control strain.
DNA Extraction and Polymerase Chain Reaction
Genomic DNA was extracted from the colonies of ESBL-producing organisms by the boiling method. The presence of TEMbla was detected by the specific primer (forward 5΄- CATTTCCGTCGCCCTTATTC -3´, reverse -3´ CGTTCATCCATAGTTGCCTGAC 5΄, Takapou Zist Company, Iran).20 The PCR was performed in a total reaction volume of 25 µL consisting 10 pmol of each primer, 5 μL DNA sample, 1.5 mM MgCl2, 0.2 mM of each dNTP, and 5 u Taq DNA polymerase (Takapou Zist Company, Iran). Moreover, the amplification was conducted by initial denaturation at 94°C for three minutes and 35 cycles, 45 seconds at 94°C, 30 seconds at 52°C and one minute at 72°C, Three minutes at 72°C was considered for the final extension and the polymerase chain reaction (PCR) products (800 bp) were detected by electrophoresis through 1.5% agarose gel.
Preparation of Cinnamon and Clove Essential Oils
The EOof C. camphorawas purchased from Barij Essence Company (Kashan, Iran).The air-dried flower bud of the clove was purchased from local retail markets and transported to the laboratory. It was identified and approved by botanists at Semnan Agriculture and Natural Resource Research Center, Iran. The dried clove buds were ground and the EO was extracted through steam distillation by using Clevenger-type apparatus and stored in dark glass containers at 4°C (15).
Preparation of Bacterial Inoculum
Stock cultures were propagated through two consecutive 24-hour growth cycles on BHI broth at 35°C. Then, the bacterial suspension was adjusted to an optical density of 0.1 at 600 nm using a spectrophotometer. One milliliter of the suspension was transferred into a tube containing nine mL of 0.1% (w/v) peptone water to prepare the successive dilutions of up to 10-6. Next, 100 µL of each dilution was aseptically transferred to plates containing BHI agar and enumerated after duplicate plating from tenfold serial dilutions, and finally, incubated at 35°C for 24 hours. The exact number of inoculated bacteria were calculated through co-culture and colony counting (20).
Determination of the Minimum Inhibitory Concentration
The broth microdilution method recommended by the CLSI was used to determine the inhibitory effect of the EOs against TEM bla producing E. coli with some modifications. To this end, 200 µL of different concentrations of cinnamon (i.e., 0, 100, 200, 400, 800, and 1600 ppm) and clove (i.e., 0, 100, 200, 400, 800, 1600, and 3200 ppm) was prepared in a 96-well microplate (300 µL capacity, round–button wells) in sterile Mueller-Hinton broth (Merck, Germany) containing 10% dimethyl sulfoxide (DMSO). In addition, 20 µL of bacterial suspension was aseptically inoculated to each test well (the final bacterial concentration was 5×105 CFU/mL bacteria per well). All experiments were performed in triplicate. A positive control containing the bacterial culture and DMSO without the EO and a negative control containing only the sterile medium were performed as well.
The contents of the wells were gently mixed with a microplate reader equipped with a shaker (BioTek® Instruments, Inc., Winooski, VT, USA) for 2 minutes and the absorbance (0 hour) was immediately read at 630 nm (OD630). Further, the plates were aerobically incubated at 35°C for 24 hours and then the absorbance (24 hours) was read again (OD630) using the microplate reader. An increase in the absorbance of ≥0.1 against 0 hour demonstrated bacterial growth and turbidity. The first single or combined concentration of the EOs without turbidity was defined as the minimum inhibitory concentration(MIC) (21).
Interaction of Clove and Cinnamon Essential Oils
The serial, two-fold dilutions of the EOs were prepared using the same solvents as in the MIC tests. For this purpose, 100 µL of each cinnamon EO dilution was added to the wells of a 96-well plate in a vertical orientation and 100 µL of each clove EO dilution was added to a horizontal orientation so that the plate would contain various concentration combinations of the 2 compounds. Then, each well was inoculated with 20 µL (the final bacterial concentration was 5×105 CFU/mL bacteria per well) of bacterial suspensions and incubated at 35°C for 24 hours. The MIC of the combined cinnamon and clove EOs was defined as described above and their interactive effect was measured by using the FIC.
Theoretically, the FIC index <1 is defined as a synergistic interactive effect of the EOs while FIC = 1, 1< FIC< 2, and FIC >2 are classified as additive, neutral, and antagonistic effects, respectively (22).
Results
Phenotypic Characterization of Antimicrobial Resistance
Nearly 91% (100) out of 110 collected samples showed positive culture results for E. coli and 88 were resistant phenotypes that were related to the ESBLs. Different detected rates of resistance to antibiotics are displayed in Figure 1. The most prevalent resistance was related to tetracycline whereas the lowest rate belonged to cefepime. Furthermore, 36.3% (32) of the isolates were confirmed as the ESBL-producing E. coli by the combined disk method.
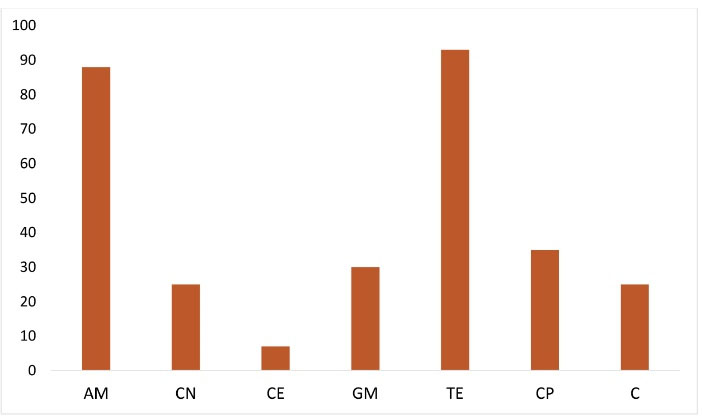
Figure 1.
Frequency of Antimicrobial Resistance Rates Among Different E. coli Strains.
Abbreviations: AM: ampicillin; CN: cefalexin; CE: cefepime; GM: gentamicin; TE: tetracycline; CP: ciprofloxacin; C: chloramphenicol.
.
Frequency of Antimicrobial Resistance Rates Among Different E. coli Strains.
Abbreviations: AM: ampicillin; CN: cefalexin; CE: cefepime; GM: gentamicin; TE: tetracycline; CP: ciprofloxacin; C: chloramphenicol.
TEM-harboring E. coli
Out of the 32 ESBL-producing strains, TEM bla gene was detected in 62.5% (20) by the PCR (Figure 2).
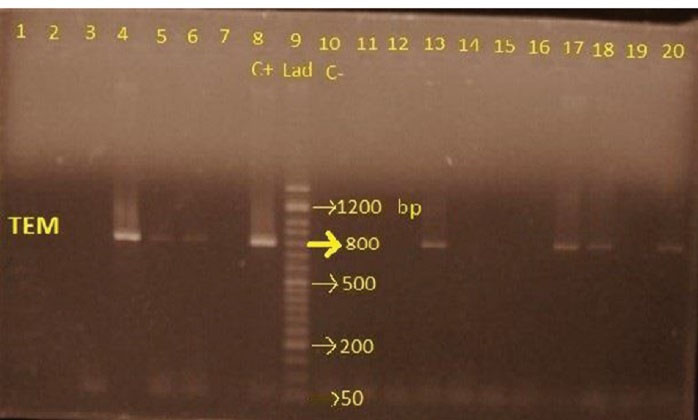
Figure 2.
Electrophoresis of PCR Products for TEM Gene.
Note. Lane 8: Positive control; Lane 10: Negative control; Lane 9: 50 bp DNA size marker; Lanes 4, 5, 6, 13, 17,18, and 20 demonstrate the 800 bp fragment of TEM gene; PCR: Polymerase chain reaction.
.
Electrophoresis of PCR Products for TEM Gene.
Note. Lane 8: Positive control; Lane 10: Negative control; Lane 9: 50 bp DNA size marker; Lanes 4, 5, 6, 13, 17,18, and 20 demonstrate the 800 bp fragment of TEM gene; PCR: Polymerase chain reaction.
Minimum Inhibitory Concentration Determination by Broth Micro Dilution Method
Table 1 presents the results of MIC determination for the cinnamon and clove EOs and a combination of these two essences against the 20 aforementioned bacterial isolates using the broth microdilution method. As shown, 17 isolates demonstrated the MIC of 1600 ppm for cinnamon and three cases shad a MIC of <1600 ppm (800, 800, and 400 ppm). The MIC of clove (EO) was 3200 ppm for 18 isolates. In addition, 2 isolates revealed a MIC of 1600 and 800 ppm. Finally, on both EOs, MIC50 for C. camphora andS. aromaticumwas observed at 1600 and 3200 ppm, respectively (Table 2).
Table 1.
The Minimum Inhibitory Concentration of Cinnamon and Clove Essential Oils on E s c h e r i c h i a co l iIsolates (ppm) in Separately and Combinatorial Approaches Determined by Using Micro Dilutions Method
|
MIC Cinnamon EssentialOil
|
MICClove Essential Oil
|
Cinnamon/Clove Essential Oil
|
Combination Effect
|
FIC Index
|
| 1600 |
3200 |
400+1600 |
Synergistic |
0.75 |
| 1600 |
3200 |
800+1600 |
Additive |
1 |
| 1600 |
3200 |
800+1600 |
Additive |
1 |
| 1600 |
3200 |
800+1600 |
Additive |
1 |
| 1600 |
3200 |
800+400 |
Synergistic |
0.62 |
| 800 |
1600 |
400+100 |
Synergistic |
0.56 |
| 1600 |
3200 |
800+800 |
Synergistic |
0.75 |
| 1600 |
3200 |
800+1600 |
Additive |
1 |
| 800 |
3200 |
400+800 |
Synergistic |
0.75 |
| 1600 |
3200 |
800+1600 |
Additive |
1 |
| 1600 |
3200 |
800+800 |
Synergistic |
0.75 |
| 1600 |
3200 |
400+1600 |
Synergistic |
0.75 |
| 1600 |
3200 |
800+800 |
Synergistic |
0.75 |
| 400 |
800 |
100+400
200+100 |
Synergistic |
0.625 |
| 1600 |
3200 |
800+1600 |
Additive |
1 |
| 1600 |
3200 |
800+1600 |
Additive |
1 |
| 1600 |
3200 |
800+1600 |
Additive |
1 |
| 1600 |
3200 |
800+1600 |
Additive |
1 |
| 1600 |
3200 |
800+1600 |
Additive |
1 |
| 1600 |
3200 |
800+1600 |
Additive |
1 |
Abbreviations:MIC, minimum inhibitory concentration; FIC: fractional inhibitory concentration.
Table 2.
The Minimum Inhibitory Concentration Ranges and MIC50
|
Essential Oil
|
MIC
s
Range (ppm)
|
MIC
50
(ppm)
|
|
Value
|
NO
|
|
Cinnamon camphora
|
400-1600 |
1600 |
17 |
|
Syzygium aromaticum
|
800-3200 |
3200 |
18 |
Determination of Fractional Inhibitory Concentration Indices
The combination effect of the EOs was investigated by calculating the FIC index. The FIC indices ranging from 0.56 to 1 are listed in Table 1. The FIC index of these two EOs in combination suggests their synergistic inhibitory effect on nine isolates and additive inhibitory effect on 11 isolates.
Discussion
Previous studies focused on the zoonotic potential risk of E. coli and the significance of antibacterial resistance transmission through the food chain caused the ESBL-producing E. coli in chickens and humans (2,4,23).
In this study, 32 (36.3%) out of 88 tested isolates were found as the ESBL-producing E. coli using the combined disk method. This was similar to the prevalence of the ESBL-producing E. coli in Korea, which was reported 41.9% and 33.3% in poultry carcasses and fecal samples, respectively (3,23).Slightly different results were obtained for the E. coli isolated from poultry in Germany (50%) (23).A lower rate of ESBL-producing E. coli was found in Zambia (4).These variations may be attributed to differences in the study design, sample size, and biosecurity management in poultry or a history of antibiotic usage on a farm.
In the present study, the prevalence of TEMbla was 62.5% whereas all the ESBL-producing E. coli in the Korean samples harbored the TEM (3) and SHVbla was the most prevalent in Germany (23).
Scientists believed that the transmission of ESBL genes from poultry to humans is most likely through the food chain (4,23,24).Therefore, the use of a safe method for combating the ESBL-producing bacteria in food is a global challenge. The application of EOs with antimicrobial potency as a flavoring bio-preservative has well documented in the last decade (10,16). However, to the best of our knowledge, this is the first report of the combined effect of cinnamon and clove EOs against TEMbla producing E. coli isolated from poultry colibacillosis.
According to the results of this study, all 20 TEMbla producing E. coli, investigated by broth microdilution assay, were sensitive to the EOs of cinnamon and clove in the range of 400 to 1600 and 800 to 3200 ppm, respectively. In all experimental strains, cinnamon had a stronger antibacterial effect compared to clove and showed a similar effect in lower concentrations. Knight and Mckeller studied several EOs and isolated the components for the antimicrobial activity against E. coli O157:H7. The results of their study indicated that the EOs of cinnamon and clove strongly inhibited the growth of E. coli O157:H7 at neutral and acidic pH (25). Similarly, Cava et al found a similar inhibitory effect of cinnamon and clove against L. monocytogenes in the pasteurized milk (26).
These effects are closely related to the antimicrobial activity of the main components of these EOs. It is assumed that the growth ofTEMbla producing E. coli was generally inhibited due to the antibacterial effects of the phytochemical components of these extracts such as cinnamaldehyde and eugenol. In another study, Saeidi et al evaluated the antibacterial activity of some natural plant extracts against the ESBL-producing E. coli isolated from the urine culture which harbors the TEM gene. The results showed that ciprofloxacin as a strong commercial choice in the treatment of urinary tract infection had the least effect against E. coli. However, all the natural plant extracts used in the study inhibited the growth of the ESBL-producing E. coli (19).
Some studies reported that aldehyde groups can cross-link with DNA and/or proteins and interfere with their normal functions. Consistent with this, cinnamaldehyde as a major compound of cinnamon inhibits different enzymes involved in cytokinesis, act as ATPase inhibitor, and disturb cell membrane in different concentrations. It also can bind to FstZ protein and prevent cell division by inhibiting its GTP-dependent polymerization (17,27).
In this regard, the antimicrobial activity of eugenol as the main constituent in clove EOs is linked to its ability to non-specific permeability through the cell membrane. Further, the hydroxyl group of eugenol can bind to proteins and inhibit some enzymes such as histidine decarboxylase, amylase, and proteinase. The other action mechanisms of cinnamon and clove EOs are based on the uptake of glucose and interfere with the proton motive force, electron flow, and active transport (26).
Although the antimicrobial activity of herbal EOs is well documented, it is considered that the high concentration of EOs should be used in food in order to obtain an optimal antimicrobial effect in the food systems as proved in the in vitro assay. However, the excessive consumption of an EO may have an adverse effect on the organoleptic properties of food or not economically valuable. The application of the EOs and/or their isolated components simultaneously is considered as a new approach for overcoming these problems. Furthermore, using combined essences with synergistic or additive effects results in the use of fewer concentrations of the same essences alone and increases the efficacy of the EOs in food systems (12,28). A synergistic effect occurs when a mixture of two EOs have a more antimicrobial activity compared to the sum of the individual components. Moreover, an additive interaction is obtained when a blend of two EOs have a combined effect equal to the sum of the individual compounds (17).Pei et al investigated the antibacterial activities of eugenol, cinnamaldehyde, thymol, carvacrol, and their combinations against E. coli and found that treatments with cinnamaldehyde/eugenol, thymol/eugenol, carvacrol/eugenol, and thymol/carvacrol have synergistic effects (29).
In the present study, the investigation of the minimal inhibitory concentration of the EOs by the FIC index showed that the combination of the EOs of cinnamon and clove have synergistic or additive interaction even against the TEM-producing E. coli.
Previous evidence revealed the mechanism of the combined effect of EOs or their purified components against microorganisms (12).
The mechanism of synergistic or additive interactions between the EOs may be due to the improvement of bioavailability or their solubility in combination with each other or targeting multiple sites in the bacterial cell. Additionally, several acceptable hypotheses are available for explaining these effects, including the sequential inhibition of some biochemical pathways, the inhibition of protective enzymes, and the enhancement of the uptake of antimicrobial agents by increasing the number and size of the pores created by binding some components to proteins in the cell membrane (17).
Based on previous research, it seems that the observed synergistic and additive interactions in this study are attributed to the eugenol and cinnamaldehyde as predominant components of clove and cinnamon. In addition, it can be explained by their contribution in membrane disruption by inhibiting ATPase activity, as well as the inhibition of the production or activity of enzymes such as protease and amylase, and finally, interference with the proton motive force (30).
In conclusion, the results of this study revealed that cinnamon and clove have antibacterial activities and thus highlighted the advantages of the combination usage of their EOs against foodborne pathogens like the ESBL-producing E. coli. However, the results only demonstrated the in vitro activity of the EOs and potential interactions between EOs and food matrices should further be investigated to prove their effects on the food systems.
Conflict of Interest Disclosures
The authors declare that they have no conflicts of interest.
Acknowledgements
The authors would like to appreciate Mrs. Reisian at the Department of Pathobiology, the Veterinary Medicine of Semnan University for her technical assistance during the procedure of the experiments.
References
- Horigan V, Kosmider RD, Horton RA, Randall L, Simons RR. An assessment of evidence data gaps in the investigation of possible transmission routes of extended spectrum beta-lactamase producing Escherichia coli from livestock to humans in the UK. Prev Vet Med 2016; 124:1-8. doi: 10.1016/j.prevetmed.2015.12.020 [Crossref] [ Google Scholar]
- Ewers C, Bethe A, Semmler T, Guenther S, Wieler LH. Extended-spectrum beta-lactamase-producing and AmpC-producing Escherichia coli from livestock and companion animals, and their putative impact on public health: a global perspective. Clin Microbiol Infect 2012; 18(7):646-55. doi: 10.1111/j.1469-0691.2012.03850.x [Crossref] [ Google Scholar]
- Lim JS, Choi DS, Kim YJ, Chon JW, Kim HS, Park HJ. Characterization of Escherichia coli-Producing Extended-Spectrum beta-Lactamase (ESBL) Isolated from Chicken Slaughterhouses in South Korea. Foodborne Pathog Dis 2015; 12(9):741-8. doi: 10.1089/fpd.2014.1921 [Crossref] [ Google Scholar]
- Chishimba K, Hang’ombe BM, Muzandu K, Mshana SE, Matee MI, Nakajima C. Detection of extended-spectrum beta-lactamase-producing Escherichia coli in market-ready chickens in Zambia. Int J Microbiol 2016; 2016:5275724. doi: 10.1155/2016/5275724 [Crossref] [ Google Scholar]
- Hasan B, Laurell K, Rakib MM, Ahlstedt E, Hernandez J, Caceres M. Fecal Carriage of Extended-Spectrum beta-Lactamases in Healthy Humans, Poultry, and Wild Birds in León, Nicaragua-A Shared Pool of blaCTX-M Genes and Possible Interspecies Clonal Spread of Extended-Spectrum beta-Lactamases-Producing Escherichia coli. Microb Drug Resist 2016; 22(8):682-7. doi: 10.1089/mdr.2015.0323 [Crossref] [ Google Scholar]
- Börjesson S, Egervärn M, Lindblad M, Englund S. Frequent occurrence of extended-spectrum beta-lactamase- and transferable AmpC beta-lactamase-producing Escherichia coli on domestic chicken meat in Sweden. Appl Environ Microbiol 2013; 79(7):2463-6. doi: 10.1128/aem.03893-12 [Crossref] [ Google Scholar]
- Founou LL, Founou RC, Essack SY. Antibiotic resistance in the food chain: a developing country-perspective. Front Microbiol 2016; 7:1881. doi: 10.3389/fmicb.2016.01881 [Crossref] [ Google Scholar]
- Aarestrup FM, Wegener HC, Collignon P. Resistance in bacteria of the food chain: epidemiology and control strategies. Expert Rev Anti Infect Ther 2008; 6(5):733-50. doi: 10.1586/14787210.6.5.733 [Crossref] [ Google Scholar]
- Khaleque MA, Keya CA, Hasan KN, Hoque MM, Inatsu Y, Bari ML. Use of cloves and cinnamon essential oil to inactivate Listeria monocytogenes in ground beef at freezing and refrigeration temperatures. LWT 2016; 74:219-23. doi: 10.1016/j.lwt.2016.07.042 [Crossref] [ Google Scholar]
- Kon KV, Rai MK. Plant essential oils and their constituents in coping with multidrug-resistant bacteria. Expert Rev Anti Infect Ther 2012; 10(7):775-90. doi: 10.1586/eri.12.57 [Crossref] [ Google Scholar]
- Shahverdi AR, Monsef-Esfahani HR, Tavasoli F, Zaheri A, Mirjani R. Trans-cinnamaldehyde from Cinnamomum zeylanicum bark essential oil reduces the clindamycin resistance of Clostridium difficile in vitro. J Food Sci 2007; 72(1):S055-8. doi: 10.1111/j.1750-3841.2006.00204.x [Crossref] [ Google Scholar]
- Bassolé IHN, Juliani HR. Essential oils in combination and their antimicrobial properties. Molecules 2012; 17(4):3989-4006. doi: 10.3390/molecules17043989 [Crossref] [ Google Scholar]
- Zouhir A, Jridi T, Nefzi A, Ben Hamida J, Sebei K. Inhibition of methicillin-resistant Staphylococcus aureus (MRSA) by antimicrobial peptides (AMPs) and plant essential oils. Pharm Biol 2016; 54(12):3136-50. doi: 10.1080/13880209.2016.1190763 [Crossref] [ Google Scholar]
- Kovács JK, Felső P, Makszin L, Pápai Z, Horváth G, Ábrahám H. Antimicrobial and Virulence-Modulating Effects of Clove Essential Oil on the Foodborne Pathogen Campylobacter jejuni. Appl Environ Microbiol 2016; 82(20):6158-66. doi: 10.1128/aem.01221-16 [Crossref] [ Google Scholar]
- Xu JG, Liu T, Hu QP, Cao XM. Chemical composition, antibacterial properties and mechanism of action of essential oil from clove buds against Staphylococcus aureus. Molecules 2016; 21(9). doi: 10.3390/molecules21091194 [Crossref]
- Johny AK, Hoagland T, Venkitanarayanan K. Effect of subinhibitory concentrations of plant-derived molecules in increasing the sensitivity of multidrug-resistant Salmonella enterica serovar Typhimurium DT104 to antibiotics. Foodborne Pathog Dis 2010; 7(10):1165-70. doi: 10.1089/fpd.2009.0527 [Crossref] [ Google Scholar]
- Hyldgaard M, Mygind T, Meyer RL. Essential oils in food preservation: mode of action, synergies, and interactions with food matrix components. Front Microbiol 2012; 3:12. doi: 10.3389/fmicb.2012.00012 [Crossref] [ Google Scholar]
- Fratini F, Mancini S, Turchi B, Friscia E, Pistelli L, Giusti G. A novel interpretation of the Fractional Inhibitory Concentration Index: The case Origanum vulgare L and Leptospermum scoparium J R et G Forst essential oils against Staphylococcus aureus strains. Microbiol Res 2017; 195:11-7. doi: 10.1016/j.micres.2016.11.005 [Crossref] [ Google Scholar]
- Saeidi S, Amini Boroujeni N, Ahmadi H, Hassanshahian M. Antibacterial activity of some plant extracts against extended-spectrum beta-lactamase producing Escherichia coli isolates. Jundishapur J Microbiol 2015; 8(2):e15434. doi: 10.5812/jjm.15434 [Crossref] [ Google Scholar]
- Dallenne C, Da Costa A, Decré D, Favier C, Arlet G. Development of a set of multiplex PCR assays for the detection of genes encoding important beta-lactamases in Enterobacteriaceae. J Antimicrob Chemother 2010; 65(3):490-5. doi: 10.1093/jac/dkp498 [Crossref] [ Google Scholar]
- Gandomi H, Abbaszadeh S, JebelliJavan A, Sharifzadeh A. Chemical Constituents, Antimicrobial and Antioxidative Effects of Trachyspermum ammi Essential Oil. J Food Process Preserv 2014; 38(4):1690-5. doi: 10.1111/jfpp.12131 [Crossref] [ Google Scholar]
- Santiesteban-López A, Palou E, López-Malo A. Susceptibility of food-borne bacteria to binary combinations of antimicrobials at selected a(w) and pH. J Appl Microbiol 2007; 102(2):486-97. doi: 10.1111/j.1365-2672.2006.03092.x [Crossref] [ Google Scholar]
- Dahms C, Hübner NO, Kossow A, Mellmann A, Dittmann K, Kramer A. Occurrence of ESBL-producing Escherichia coli in livestock and farm workers in Mecklenburg-Western Pomerania, Germany. PLoS One 2015; 10(11):e0143326. doi: 10.1371/journal.pone.0143326 [Crossref] [ Google Scholar]
- Leverstein-van Hall MA, Dierikx CM, Cohen Stuart J, Voets GM, van den Munckhof MP, van Essen-Zandbergen A. Dutch patients, retail chicken meat and poultry share the same ESBL genes, plasmids and strains. Clin Microbiol Infect 2011; 17(6):873-80. doi: 10.1111/j.1469-0691.2011.03497.x [Crossref] [ Google Scholar]
- Knight KP, McKellar RC. Influence of cinnamon and clove essential oils on the D- and z-values of Escherichia coli O157:H7 in apple cider. J Food Prot 2007; 70(9):2089-94. doi: 10.4315/0362-028x-70.9.2089 [Crossref] [ Google Scholar]
- Cava R, Nowak E, Taboada A, Marin-Iniesta F. Antimicrobial activity of clove and cinnamon essential oils against Listeria monocytogenes in pasteurized milk. J Food Prot 2007; 70(12):2757-63. doi: 10.4315/0362-028x-70.12.2757 [Crossref] [ Google Scholar]
- Shen S, Zhang T, Yuan Y, Lin S, Xu J, Ye H. Effects of cinnamaldehyde on Escherichia coli and Staphylococcus aureus membrane. Food Control 2015; 47:196-202. doi: 10.1016/j.foodcont.2014.07.003 [Crossref] [ Google Scholar]
- Brandt AL, Castillo A, Harris KB, Keeton JT, Hardin MD, Taylor TM. Inhibition of Listeria monocytogenes by food antimicrobials applied singly and in combination. J Food Sci 2010; 75(9):M557-63. doi: 10.1111/j.1750-3841.2010.01843.x [Crossref] [ Google Scholar]
- Pei RS, Zhou F, Ji BP, Xu J. Evaluation of combined antibacterial effects of eugenol, cinnamaldehyde, thymol, and carvacrol against E coli with an improved method. J Food Sci 2009; 74(7):M379-83. doi: 10.1111/j.1750-3841.2009.01287.x [Crossref] [ Google Scholar]
- Langeveld WT, Veldhuizen EJ, Burt SA. Synergy between essential oil components and antibiotics: a review. Crit Rev Microbiol 2014; 40(1):76-94. doi: 10.3109/1040841x.2013.763219 [Crossref] [ Google Scholar]