Avicenna Journal of Clinical Microbiology and Infection. 6(3):77-82.
doi: 10.34172/ajcmi.2019.13
Research Article
To Study In Vitro Anti-proliferative and Pro-apoptotic properties of Salmonella typhi in Human Pancreatic Cancer Cell Line
Amir Khodavirdipour 1  , Hamideh Rouhani nejad 2, *
, Hamideh Rouhani nejad 2, *  , Masoud Zandi 3, Adineh Khodavirdipour 3, Nazanin Khayyam 4
, Masoud Zandi 3, Adineh Khodavirdipour 3, Nazanin Khayyam 4
Author information:
1Division of Human Genetics, Department of Anatomy, St. John’s Hospital, Bangalore, India
2Department of Microbiology, Faculty of Science, Islamic Azad University, Hamedan, Hamedan, Iran
3Department of Biotechnology and Bioscience, Malek-Ashtar University of Technology, Tehran, Iran
4Faculty of Biological Sciences,North Tehran Branch, Islamic Azad University, Tehran, Iran
Abstract
Background: Pancreatic cancer is one of the most common types of cancer that affects the gastrointestinal tract and the induction of apoptosis in cancer cells is generally one of the suitable methods for treating cancer. Salmonella typhi induces apoptosis by activating the caspase pathway.
Methods: This experimental study was conducted on a standard strain and the bacteria were cultured on an SS-Agar medium. Then, sonication was used to isolate bacterial proteins and ZellBio was utilized to determine the concentration of such proteins. In addition, 1 million cells per well were cultivated in the cell culture plate in order to treat the cell line with bacteria and to examine the expression of the Bax and Bcl-2 genes. Then, RNA was extracted and cDNA was synthesized after 24 hours of incubation. The Bax and Bcl-2 genes were expressed by Real-time polymerase chain reaction (RT-PCR).
Results: The findings confirmed the role of Salmonella typhi proteins in inducing apoptosis in a pancreatic cancer cell. In other words, the concentration of 10 mg Salmonella typhi proteins increased the expression of the Bax gene while reducing the expression of the Bcl-2 gene.
Conclusions: Overall, bacterial treatments have unique mechanisms for treating cancer. For example, bacteria can specifically target the tumors, actively penetrate into the tissue, and potentially form a controlled form of toxicity. Accordingly, further studies are recommended to investigate the role of bacteria, especially Salmonella in the treatment of cancer cells.
Keywords: Salmonella typhi, RT-PCR, Pancreatic cancer, Bacterial-induced Apoptosis, Cancer treatment
Copyright and License Information
© 2019 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Background
Cancer can be defined as uncontrolled cell division under the influence of genetic and/or epigenetic factors such as environment and lifestyle (1,2). Pancreas usually forms cellular mass when the pancreas defected cells continue to divide and scape the programmed cell death. This recently formed tumor can become metastasis as well (3,4). In addition, pancreatic cancer prevalence is higher in the elderly and around half of these people are above 75 years of age. However, this type of cancer is seldom observed in people under 40. Gender specificity is off the table in this cancer and both genders have an equal chance of catching pancreas cancer. There is no obvious sign and the symptoms are tumorigenesis at the early stages of this cancer, therefore, it is one of the main reasons which makes the early diagnostics almost impossible (4). Data analysis suggests that most of the cancers happen to be the exocrine pancreatic part and their most common symptoms are back pain, abdominal cramps, jaundice, and weight loss (5). By considering the aggressive nature of cancer yet complex mechanisms, conventional treatment options are surgery, as well as chemo and radiotherapy, but they are short-time alternatives and mostly less effective or even ineffective. Several drawbacks of the above-mentioned methods include the lack of specificity, the presence of numerous side effects, and the chances of metastasis. Thus, finding an effective method enhances the specificity and minimize the side effects for greater results, suppressing the uncontrolled growth (6,7).
Since the early 1990s, proteins, bacterial toxins, and the like came to light as a potential treatment for cancer (8). So far, researchers have identified more than 100 genes which play an undeniable role as the protector or guardian of the cell and its longevity. Apoptosis is a mechanism for excreting defected and unhealthy cells in eukaryotes including the human. Further, the suppression of apoptosis is a fundamental factor in human malignancies. Cancer cells can escape apoptosis which will turn the cell into the fetal accumulated mass of cells which are known as the tumor. Two large families of genes are involved in the apoptosis pathway, namely, Bcl-2 and caspases. Bcl-2 protein plays a dual role either pro or anti-apoptosis. Similarly, Mcl-1, Bcl- 2 , and Bcl-xL association has an anti-apoptosis impact while proteins like Bax, Bak, Bad, and Bim are the key players in the pro-apoptosis side (9,10). The induction of apoptosis is one of the most well-known options to consider in cancer treatment since it has the minimum risk including less side effects and a plus point like higher specificity. Researchers are in a race against time for finding the best compound to nail the goal. These compounds can be either the extracts of medicinal plants or inorganic compounds. As previously mentioned, the blooming idea of bacterial-mediated apoptosis has come under the spotlight since almost three decades ago and many advances have so far been made in this regard. Salmonella is one of those successful attempts which can be a prominent choice in cancer treatment, along with its pathogenicity (11). Furthermore, Salmonella belongs to Enterobacteriaceae but it is simultaneously considered as the gut bacterium of humans and animals (12). Salmonella typhi and Salmonella Paratyphi are the main pathogenesis of typhoid fever (13). The gram-negative bacilli are non-spore-forming, which are encapsulated and motile. Salmonella is regarded as a facultative anaerobe as well (14). Previous studies have rarely investigated bacterial-mediated apoptosis induction on pancreatic cell lines compared to the other cancer cell lines. Pancreatic cancer by 338 000 new cases per year is the 12th pervasive type of cancer. Accordingly, for this study, the pancreatic cancer cell line was chosen to evaluate its effectiveness, measure the expression ratio of Bax/Bcl2 by the latest and sensitive methods (e.g., real-time polymerase chain reaction and the like), and thus assess the outcome and elucidate how this gram-negative bacterium can alter and affect the apoptosis pathway.
Materials and Methods
Sample Procurement
In this experimental study, the Aspc-1 cell line and ATCC certified Salmonella typhi bacteria were purchased from the Iran Biological Resource Center and the Pasteur Institute of Iran. Then, a biochemical assay was performed to assess the purity of the obtained species.
Obtaining Bacterial Protein
To this end, 2 µL of sterile PBS buffer was added to a 5 mL bacterial pellet and sonicate at 250 W intensity for 5 minutes with 30-second intervals. Next, the mixture was centrifuged at 8°C at 9000 rpm for 10 minutes. Then, the supernatant was harvested by a sterile syringe, followed by filtering and transferring it into a micro tube and storing it at -20°C. Eventually, protein concentration was analyzed by Bradford assay (ZellBio).
MTT Assay
The Aspc-1 cell line was cultured overnight in RPMI (Sigma-Aldrich, Germany) supplemented with 10% FBS (Gibco, Grand Island, NY), 1% penicillin (100 U/mL), and streptomycin (100 g/mL, Biosera, UK) and then were incubated at 37ºC in an atmosphere containing 5% CO2 and 100% humidity. The medium was replaced with the fresh one in every 48 hours before any treatment aiming at covering the surface of the flask for 80% by the culture cells. Next, Aspc-1 cells were seeded on six-well plates at a density of 1.0×106 cells/well leave first well for untreated (control) and the remaining plates/cells were filled up with different concentrations of bacterial extract (i.e., 10 and 100 µg, along with 1, 5, and 10 mg). After 24 hours of incubation, the cells were washed 3 times with PBS and all media were replaced by fresh media, followed by the addition of MTT reagent to each well. The cells were further cultured for 4 hours and then all of the media was replaced with dimethyl sulfoxide. After 30 minutes of incubation, the six-well plates were shaken strongly and the absorbance was measured in an Elisa Reader (SpectraMax M2e, Molecular Devices) at 570 nm.
Real-Time Polymerase Chain Reaction (RT-PCR)
The qRT-PCR was performed on Aps1 cells for 24 hours in order to determine the effect of the extract of Salmonella typhi on the mRNA expression of Bcl-2 and Bax. After treatment, the contents of each well were transferred to a micro tube and stored at -20°C. Later, 200 µL of chloroform was added to each 1.5 mL micro tube and it was centrifuged at 12000 rpm for 5 minutes at 8°C, followed by obtaining three phases of phenol, protein, and RNA. The supernatant was relocated to a new micro tube and top up with isopropanol and kept at -20°C for 12 hours. Next, it was centrifuged at 12000 rpm for 15 minutes at 8°C after 12 hours. In addition, the supernatant was discarded and the remaining alcohol was let to evaporate and air dry. Finally, 30 µL DEPC-treated water was added to the micro tube. The agarose gel electrophoresis was performed to confirm the RNA extraction as well. Further, the random hexamer primer kit (Thermoscience, Lithuania, EU) was utilized as the protocol of per kit for DNA synthesis. Similarly, one-step RT-PCR was performed on by real-time PCR (Applied biosystem-USA) after the extraction of total RNA, and the reconfirmation of gene expression with RT-PCR. RT-PCR evaluation for all genes is listed in Table1. For target sequence amplifications, 100 ng (5 µL) of RNA was used per 2 µL reaction volume as well. For each sample, the reference gene (β-actin) and the target genes were amplified in the same run, followed by obtaining standard curves using the logarithmic dilution series of total RNA. Eventually, PCR products were interpreted and analyzed by the REST software and the results of less than < 0.05 were considered as desired outcomes.
Table 1.
Primer Sequenced Used in Real-time-PCR
|
Gene
|
Primer Sequence
|
Melting Temperature (Tm)
|
PCR Product Size (bp)
|
|
Bax
|
Forward |
5'- TGGGCTGGACATTGGACTTCC -3' |
61.77 |
173 |
| Reverse |
5'- GCCTCAGCCCATCTTCTTCC -3' |
60.39 |
|
Bcl-2
|
Forward |
5'- ACTTGACAGAGGATCATGCTG-3' |
62.31 |
169 |
| Reverse |
5'- ACTTGATTCTGGTGTTTCCC -3' |
62.96 |
|
Beta-actin
|
Forward |
5'-TCCTCCTGAGCGCAAGTAC-3' |
62.10 |
155 |
| Reverse |
5'-CCTGCTTGCTGATCCACATCT-3' |
60.34 |
Abbreviation:PCR, polymerase chain reaction.
Results
Apoptosis Analysis
The existence frequency of Aps-1 cells was calculated by incubating the cells with different concentrations of bacteria protein extract (24 hours). The protein extract could prevent the proliferation of Aps-1 cells at concentrations of 5 and 10 mg/mL. Figure 1 displays dose-response behavior against the cells.

Figure 1.
Pancreatic Cancer Cell Line Treated With Different Dosages of Salmonella typhi Bacterial Extract.
.
Pancreatic Cancer Cell Line Treated With Different Dosages of Salmonella typhi Bacterial Extract.
mRNA Expression of Selected Apoptosis-Related Gene Transcripts
The formation of 16 sRNA and 28 sRNA bands confirmed that RNA extraction and electrophoresis were performed correctly (Figure 2).
Based on data analysis and interpretation, the expression ratio of genes in the treated samples by 10 and 100 µgm showed no significant changes compared to the control group (P > 0.05). On the other hand, samples treated by higher doses (e.g., 5 and 10 mg) of Salmonella typhi protein demonstrated up-regulation in Baxgene (P < 0.05). The gene expression amount at 5 mg was 1.905 whereas the highest expression changes was related to 10 mg concentration of Salmonella typhi bacterial protein which was equal to 11.551. The gradual expression of Bcl-2 gene at 10 µg treated samples represented negligible changes in comparison to the β-actin gene expression of the control sample. Furthermore, no noticeable changes were found (P > 0.05) at 100 µg and 1 mg of Salmonella typhi concentrations. The highest amount of decline in the down-regulation of the Bcl-2 anti-apoptosis gene belonged to 5 mg concentration of bacterial extract by the mean value of 0.352.
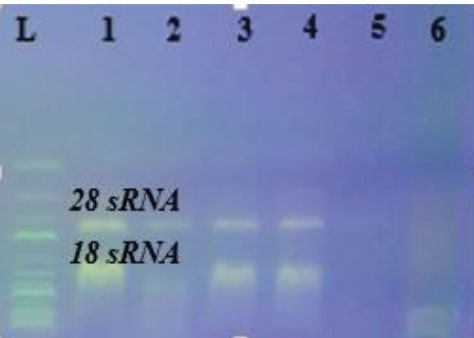
Figure 2.
The Resultof Salmonella typhi RNA Extract (R ladder 1 kb).
.
The Resultof Salmonella typhi RNA Extract (R ladder 1 kb).
As illustrated in Figure 3, the up-regulation of Baxgene was more than the other genes, which confirmed the positive apoptosis induction and incline in the rate of the programmed cell death. Moreover, Bcl-2 -5 was another gene with significant changes is. The down-regulation of this gene increased the apoptosis by considering the anti-apoptotic properties of the Bcl-2 gene. However, there were no such remarkable changes in the expression of Bax1, Bcl-2 -1, and Bcl-2 -2. Additionally, no suggestive changes were observed in the other involved genes compared to the control ones. Therefore, 10 mg concentration of the bacterial extract used to treat the pancreatic cancer cell line enhanced the apoptosis rate and thus suppressed the growth of cancer cells. furthermore, up-regulation and down-regulation of Bax and Bcl2 genes shown in figure 4 and 5 respectively.
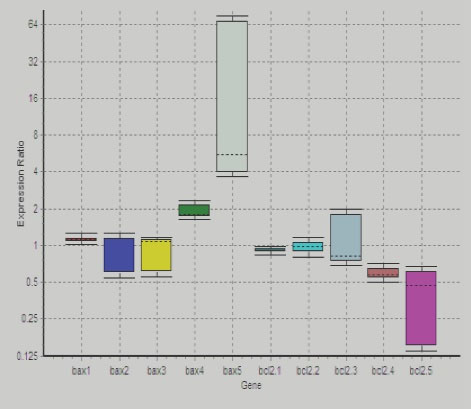
Figure 3.
X Axis Showing Different Baxes Stands for 50% of the Median of the Observed Dots Partitioning Between Gene ExpressionShadows Representing the Minimum and MaximumRate.
.
X Axis Showing Different Baxes Stands for 50% of the Median of the Observed Dots Partitioning Between Gene ExpressionShadows Representing the Minimum and MaximumRate.
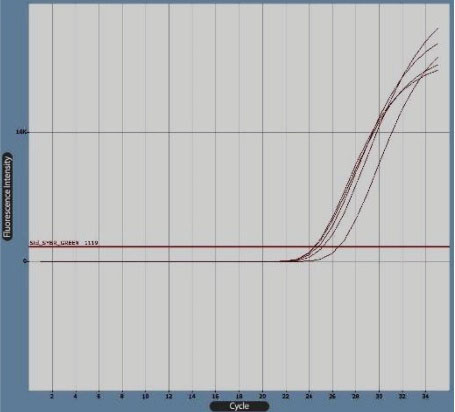
Figure 4.
Bcl-2 Gene Boost Diagram in Real-Time Polymerase Chain Reaction by Cyber Green Fluorescence. Note. The diagram was produced by LightCycler software.
.
Bcl-2 Gene Boost Diagram in Real-Time Polymerase Chain Reaction by Cyber Green Fluorescence. Note. The diagram was produced by LightCycler software.
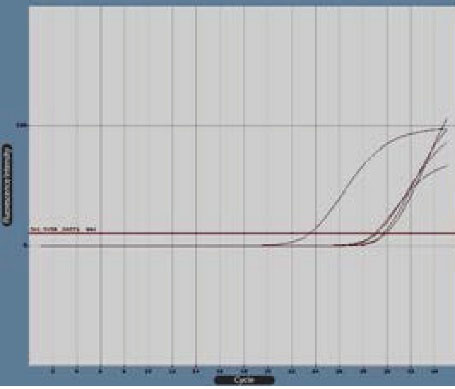
Figure 5.
Bax Gene Boost Diagram in Real-Time Polymerase Chain Reaction by Cyber Green Fluorescence. Note. The diagram was created by LightCycler software.
.
Bax Gene Boost Diagram in Real-Time Polymerase Chain Reaction by Cyber Green Fluorescence. Note. The diagram was created by LightCycler software.
Discussion
The prevalence of pancreatic cancer has demonstrated an increase in recent years and, according to the World Cancer Research Fund International, its mortality ranks 12 after lung, breast, colorectal, prostate, stomach, liver, cervix, oesophagus, bladder, non-Hodgkin lymphoma, and leukaemia. Apoptosis follows unique yet complex mechanisms to nail the programmed cell death without inflammation, unlike necrosis. In addition, apoptosis is generally defined as the removal of unwanted or defected cells in eukaryotes or even some prokaryotes. Nowadays, scientists employ apoptosis inducer or occasionally apoptosis suppresser genes in in vitro studies in order to direct the immune system toward the intended pathway regarding establishing the balance between cellular and humoral immunity responses. Further investigation continues in vivo to expand the horizon and evaluate the chances for clinical trials (16). The results of the current study are in line with previous findings and confirm the positive role of Salmonella typhi bacterial extract in the induction of apoptosis. For example, Raymond et al. studied mitochondrial-mediated apoptosis which activates caspase pathways by a bacterium like Salmonella typhi and reported significantly decisive results (17,18). In addition, this bacterium is found to affect Bax/Bcl-2 gene expression, directly or indirectly (19). Likewise, the effect of Salmonella typhi bacterial extract was undeniable on the expression of the above-mentioned genes in our study. Further, Chorobik et al, by analyzing all species of Salmonella in spite of their pathogenicity, as well as considering their role in triggering mitochondrial-dependant intrinsic pathway could nominate Salmonella as a potential treatment option in some cancers (20). Another study on the remedial properties of Salmonella in China demonstrated that this bacterium can activate exclusive mechanisms to achieve its apoptotic purposes (21). Likewise, our findings suggested that we can employ this property against cancer and rely on in therapy by suppressing the pathogenic factors, and subsequently, diminishing cytotoxicity. The results further proved that bacterial protein sparks off apoptosis pathways by affecting the expression of Baxand Bcl-2 genes. The first trials have been performed on the tumor-fighting properties of Salmonella species since the early 1990s, and recently, few species of Salmonella like VNP20009, A1-R, and CRC2631 have received all the approvals for use in chemotherapy (22). While studying the embryonic fibroblast (inoculated to Salmonella typhimurium) of the rat, Ruan et al found that TRAF6 (TNF receptor-associated factor) could increase the apoptosis rate by ROS accumulation, which gradually led to an elevation in Bax/Bcl-2 ratio in the mitochondrial membrane and released cytochrome C to the cytoplasm (23). Furthermore, proteins such as Bcl-2 play a crucial role in the permeability of mitochondrial outer membrane in the cells and can even modify the dynamics although its molecular mechanisms are still not fully known (24). Bcl-2 protects the cell by suppressing the apoptosis pathways (25) while the Baxgene regulates the apoptosis (26).
Our findings indicate that the bacterial protein of Salmonella typhi induces the apoptosis in the pancreatic cancer cell line. Based on the recent trials in Massachusetts in the United States, there is a possibility of employing a few types of bacteria together in cancer treatment (16). In the field of bacterial-mediated apoptosis, such bacteria can regulate a couple of unique pathways and speed up the apoptosis mechanism. The evidence further suggested that some of the infectious bacteria in certain measured doses can be used in the therapies by initiating apoptosis (18). Similarly, Camacho et al studied engineered Salmonella as the internal factory of a cell to excrete defected tumor cells. They reported that it is necessary to provisionally separate the produced proteins from the release proteins to effectively fight back against the tumor cells (27). Likewise, the extraction and separation of the bacterial protein of Salmonella typhi in the present study resulted in the induction of apoptosis in the pancreatic cancer cell line. Thus, our results confirm the key role of the bacterial protein of Salmonella typhi in the pancreatic cancer cell line. The above-mentioned extract has a remarkable influence on the Bax/Bcl-2 ratio and their expression. As mentioned earlier, Bax(prop apoptotic gene) and Bcl-2 (anti-apoptotic gene) genes up-regulate and down-regulated by 10 mg of sonicated bacterial protein, respectively. Apoptotic induction is considered a beneficial and useful method for removing the defected tumor and cancerous cells since the tumoral cells normally lack the apoptosis machinery or are somehow out of order. Thus, compounds like Salmonella typhi bacterial extract which can induce apoptosis, are extremely important since they act with higher specificity compared to radio or chemotherapy and have less known side effects. Bacterial products such as protein, enzyme, immunotoxins, and secondary metabolites are utilized since they exclusively target and remove cancerous cells either by their anti-proliferative characters, cell cycle arrest, or apoptosis induction. The recent investigation supports the idea of using the Bax/Bcl-2 measurement ratio as potential biomarkers in cancer prognosis or even prediction (28). The expression of pro and anti-apoptosis genes like Baxand Bcl-2 in combination were found highly predictive and beneficial in colorectal cancer as compared to each gene expression separately (29,30).
Conclusions
Finally, we suggest further anti-tumoral compositions in this compound, therefore, future studies can be in vivo and probably human clinical trials. Based on the increasing rate of cancer worldwide and the burden of treatment expenses on individuals and governments, there is a growing need for such a cheap, handy, and conventional predictive method such as cancer biomarkers.
Conflict of Interests
The authors declare that there is no conflict of interest regarding the publication of this article.
Funding
None.
Acknowledgments
This research received no special grant from funding agencies in the public, commercial, or not-for-sale sector.
References
- Bond-Smith G, Banga N, Hammond TM, Imber CJ. Pancreatic adenocarcinoma. BMJ 2012; 344:e2476. doi: 10.1136/bmj.e2476 [Crossref] [ Google Scholar]
- Noori-Daloii MR, Eshaghkhani Y. lncRNAs roles in cancer occurrence. Medical Science Journal of Islamic Azad Univesity Tehran Medical Branch 2015; 25(3):163-82. [ Google Scholar]
- Burns WR, Edil BH. Neuroendocrine pancreatic tumors: guidelines for management and update. Curr Treat Options Oncol 2012; 13(1):24-34. doi: 10.1007/s11864-011-0172-2 [Crossref] [ Google Scholar]
- Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med 2014; 371(11):1039-49. doi: 10.1056/NEJMra1404198 [Crossref] [ Google Scholar]
- Wolfgang CL, Herman JM, Laheru DA, Klein AP, Erdek MA, Fishman EK. Recent progress in pancreatic cancer. CA Cancer J Clin 2013; 63(5):318-48. doi: 10.3322/caac.21190 [Crossref] [ Google Scholar]
- Anand P, Kunnumakkara AB, Sundaram C, Harikumar KB, Tharakan ST, Lai OS. Cancer is a preventable disease that requires major lifestyle changes. Pharm Res 2008; 25(9):2097-116. doi: 10.1007/s11095-008-9661-9 [Crossref] [ Google Scholar]
- Tang C, Ang BT, Pervaiz S. Cancer stem cell: target for anti-cancer therapy. FASEB J 2007; 21(14):3777-85. doi: 10.1096/fj.07-8560rev [Crossref] [ Google Scholar]
- Tangney M. Gene therapy for cancer: dairy bacteria as delivery vectors. Discov Med 2010; 10(52):195-200. [ Google Scholar]
- Melka MG, Rings F, Hölker M, Tholen E, Havlicek V, Besenfelder U. Expression of apoptosis regulatory genes and incidence of apoptosis in different morphological quality groups of in vitro-produced bovine pre-implantation embryos. Reprod Domest Anim 2010; 45(5):915-21. doi: 10.1111/j.1439-0531.2009.01463.x [Crossref] [ Google Scholar]
- Badr H, Bongioni G, Abdoon AS, Kandil O, Puglisi R. Gene expression in the in vitro-produced preimplantation bovine embryos. Zygote 2007; 15(4):355-67. doi: 10.1017/s096719940700431 [Crossref] [ Google Scholar]
- Wrenzycki C, Herrmann D, Lucas-Hahn A, Lemme E, Korsawe K, Niemann H. Gene expression patterns in in vitro-produced and somatic nuclear transfer-derived preimplantation bovine embryos: relationship to the large offspring syndrome?. Anim Reprod Sci 2004; 82-83:593-603. doi: 10.1016/j.anireprosci.2004.05.009 [Crossref] [ Google Scholar]
- Baron S Yaeger RG. Protozoa: Structure, Classification, Growth, and Development. In: Baron S, ed. Medical Microbiology. Galveston (TX): University of Texas Medical Branch at Galveston; 1996.
- Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull World Health Organ 2004; 82(5):346-53. [ Google Scholar]
- Marathe SA, Lahiri A, Negi VD, Chakravortty D. Typhoid fever & vaccine development: a partially answered question. Indian J Med Res 2012; 135:161-9. [ Google Scholar]
- Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res 2002; 30(9):e36. doi: 10.1093/nar/30.9.e36 [Crossref] [ Google Scholar]
- Forbes NS. Engineering the perfect (bacterial) cancer therapy. Nat Rev Cancer 2010; 10(11):785-94. doi: 10.1038/nrc2934 [Crossref] [ Google Scholar]
- Xiong S, Mu T, Wang G, Jiang X. Mitochondria-mediated apoptosis in mammals. Protein Cell 2014; 5(10):737-49. doi: 10.1007/s13238-014-0089-1 [Crossref] [ Google Scholar]
- Zheng JH, Min JJ. Targeted cancer therapy using engineered Salmonella typhimurium. Chonnam Med J 2016; 52(3):173-84. doi: 10.4068/cmj.2016.52.3.173 [Crossref] [ Google Scholar]
- Raymond B, Young JC, Pallett M, Endres RG, Clements A, Frankel G. Subversion of trafficking, apoptosis, and innate immunity by type III secretion system effectors. Trends Microbiol 2013; 21(8):430-41. doi: 10.1016/j.tim.2013.06.008 [Crossref] [ Google Scholar]
- Chorobik P, Czaplicki D, Ossysek K, Bereta J. Salmonella and cancer: from pathogens to therapeutics. Acta Biochim Pol 2013; 60(3):285-97. [ Google Scholar]
- Li B, He H, Zhang S, Zhao W, Li N, Shao R. Salmonella typhimurium strain SL7207 induces apoptosis and inhibits the growth of HepG2 hepatoma cells in vitro and in vivo. Acta Pharm Sin B 2012; 2(6):562-8. doi: 10.1016/j.apsb.2012.10.006 [Crossref] [ Google Scholar]
- Wang CZ, Kazmierczak RA, Eisenstark A. Strains, mechanism, and perspective: Salmonella-based cancer therapy. Int J Microbiol 2016; 2016:5678702. doi: 10.1155/2016/5678702 [Crossref] [ Google Scholar]
- Ruan H, Zhang Z, Tian L, Wang S, Hu S, Qiao JJ. The Salmonella effector SopB prevents ROS-induced apoptosis of epithelial cells by retarding TRAF6 recruitment to mitochondria. Biochem Biophys Res Commun 2016; 478(2):618-23. doi: 10.1016/j.bbrc.2016.07.116 [Crossref] [ Google Scholar]
- Bleicken S, Hofhaus G, Ugarte-Uribe B, Schroder R, Garcia-Saez AJ. cBid, Bax and Bcl-xL exhibit opposite membrane remodeling activities. Cell Death Dis 2016; 7:e2121. doi: 10.1038/cddis.2016.34 [Crossref] [ Google Scholar]
- Song HY, Deng XH, Yuan GY, Hou XF, Zhu ZD, Zhou L. Expression of bcl-2 and p53 in induction of esophageal cancer cell apoptosis by ECRG2 in combination with cisplatin. Asian Pac J Cancer Prev 2014; 15(3):1397-401. doi: 10.7314/apjcp.2014.15.3.1397 [Crossref] [ Google Scholar]
- Li Y, Zhang S, Geng JX, Hu XY. Curcumin inhibits human non-small cell lung cancer A549 cell proliferation through regulation of Bcl-2/Bax and cytochrome C. Asian Pac J Cancer Prev 2013; 14(8):4599-602. doi: 10.7314/apjcp.2013.14.8.4599 [Crossref] [ Google Scholar]
- Camacho EM, Mesa-Pereira B, Medina C, Flores A, Santero E. Engineering Salmonella as intracellular factory for effective killing of tumour cells. Sci Rep 2016; 6:30591. doi: 10.1038/srep30591 [Crossref] [ Google Scholar]
- Sturm I, Köhne CH, Wolff G, Petrowsky H, Hillebrand T, Hauptmann S. Analysis of the p53/BAX pathway in colorectal cancer: low BAX is a negative prognostic factor in patients with resected liver metastases. J Clin Oncol 1999; 17(5):1364-74. doi: 10.1200/jco.1999.17.5.1364 [Crossref] [ Google Scholar]
- Zeestraten EC, Benard A, Reimers MS, Schouten PC, Liefers GJ, van de Velde CJ. The prognostic value of the apoptosis pathway in colorectal cancer: a review of the literature on biomarkers identified by immunohistochemistry. Biomark Cancer 2013; 5:13-29. doi: 10.4137/bic.s11475 [Crossref] [ Google Scholar]
- Khodapasand E, Jafarzadeh N, Farrokhi F, Kamalidehghan B, Houshmand M. Is Bax/Bcl-2 ratio considered as a prognostic marker with age and tumor location in colorectal cancer?. Iran Biomed J 2015; 19(2):69-75. [ Google Scholar]